-
Potential for post-combustion capture CCS in glass manufacturing
Date posted:
-
-
-
Post Author
Greg Kelsall
-
-
![]()
In a recent interview with Glass International, C‑Capture Ltd described how its post-combustion carbon capture process could be a good fit with decarbonising ‘foundation industries’ such as glass and cement manufacturing. Carbon capture offers a crucial decarbonisation route for these industries where carbon emissions are inherent to their processes and cannot be easily addressed by other means (such as energy efficiency improvements, fuel switching or electrification). In order for these industries to decarbonise, they must have access to a carbon capture technology which is both effective and economic.
UK-based C-Capture has patented what it believes will be a low‑cost post‑combustion capture technology which uses up to 40% less energy than current commercially-available amine-based technologies.
Carbon capture in glass manufacturing is challenging due to the high levels of impurities found in the flue gases produced, particularly SOx and NOx emissions. The current state‑of‑the‑art technologies for carbon capture rely on amine‑based solvents which were initially developed in the 1930s. Whilst undoubtedly an effective technology in many respects, amine‑based solvents have some challenges including:
- Energy intensive regeneration
- Susceptibility to degradation, especially under harsh conditions
- High levels of corrosivity
- Potential human or environmental toxicity.
Taken together, these factors may have deterred their widespread adoption, particularly in challenging applications such as glass manufacturing. C‑Capture’s proprietary solvents do not contain amines, being based instead on a fundamentally different underlying chemistry which offers numerous benefits including reduced energy input, high stability under all conditions evaluated thus far, low corrosivity and minimal production of hazardous by‑products. C-Capture has been testing its solvents to expand the dataset and knowledge of solvent performance under challenging conditions, including high levels of SOx and NOx, alongside comparable levels of oxygen and thermal degradation as described below.
Experimental
Four solvent-ageing systems to analyse C-Capture’s capture solvent were designed, built and commissioned. Solvent-ageing experiments were then carried out under condition of:
- 140°C, nitrogen atmosphere
- 140°C, nitrogen/air (balance ~2000ppm oxygen)
- 140°C, nitrogen/sulphur dioxide (2000ppm)
- 140°C, nitrogen/nitric oxide (2000ppm).
Experiments were carried out over a two month period, with suspected degradation products analysed to provide a comparison across the different sets of conditions.
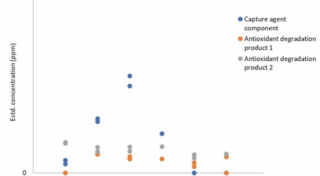
Thermal degradation: C-Capture’s proprietary CO2 capture solvent was aged at 140°C under a pressurised nitrogen atmosphere (5bar). As shown in Figure 1, minimal (<70ppm total) levels of suspected degradation products were detected throughout the experimental period, suggesting overall solvent stability is high under these conditions. Of these products, three were identified, comprising a component of the capture agent and two degradation products of the antioxidant included in the solvent (>95% of the antioxidant remained in its initial form). No clear trend in concentration was found and therefore there is insufficient evidence at present to characterise any degradation rate.
![]()
Figure 1: Impurities observed under a nitrogen atmosphere
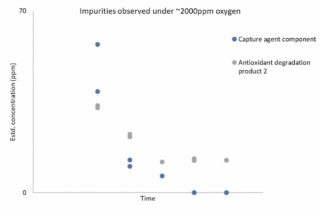
Degradation in the presence of O2: C-Capture’s solvent was tested in the presence of approximately 2000ppm of oxygen. As shown in Figure 2, a slightly higher level of suspected degradation products was initially observed, which declined to around 10ppm by the end of the experiment. Again, the overall level of observed degradation was very low and the evidence is that the solvent appears highly robust under these conditions.
![]()
Figure 2: Impurities observed under ~2000ppm oxygen
Degradation in the presence of SO2: C-Capture’s solvent was aged in the presence of 2000ppm SO2, representing the high levels of sulphur oxides found in glassmaking and other foundation industry flue gases. Very low levels of suspected degradation products were observed, reducing to below the limit of detection (~1ppm) at the end of the experiment.
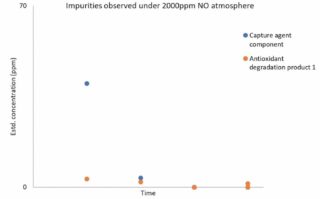
Degradation in the presence of NO: C-Capture’s solvent was aged in the presence of 2000ppm NO, representing the high levels of nitrogen oxides also found in glassmaking flue gases. As shown in Figure 3, levels of suspected degradation products were once again very low, providing further support to C-Capture’s claims regarding the robustness of its solvent under harsh conditions.
![]()
Figure 3: Impurities observed under 2000ppm NO
In conclusion, whilst these results are preliminary, they appear to be promising, demonstrating the feasibility of deploying C-Capture solvent technology in challenging conditions, such as those found in glass manufacture.
However, further development of the C-Capture is required to move towards commercialisation. As noted in another IFRF blogpost this week, MHI’s KS21 amine-based approach has been selected for the BECCS project at Drax, being a more commercially-available technology. C-Capture solvents have also been tested at Drax, as described in a previous IFRF blogpost.