-
How do I estimate the heat released by combustion of a carbonaceous particle and the heat it loses to the surroundings?
Date posted:
-
-
Post Author
dev@edge.studio
1. Background
There are a number of situations where it is required to know the rate at which heat is generated by combustion in a burning [GLOSS]carbonaceous[/GLOSS] particle as well as the rate at which the particle exchanges heat with its surroundings. The balance between these two will determine the time temperature history of the particle, and this in turn affects processes such as [GLOSS]char[/GLOSS] burnout, [GLOSS]slag[/GLOSS] formation and particle combustion stability.
A particular example of the way in which these two parameters may be used to evaluate an [GLOSS]ignition[/GLOSS] criterion for carbonaceous particles was described in a linked Combustion File (CF257).
Examples are given below of two sets of equations which may be used to calculate the rate of heat generation by the combustion reaction Qcomb, and the rate of heat exchange with the surroundings: Qloss. The equations apply to a single spherical porous particle of carbon, in the presence of oxygen.
2. Heat released by the combustion reaction: Qcomb[W]
The rate of heat release by the oxidation reaction of a carbonaceous particle Qcomb (W) can be determined from equation (1) using the additional relationships given in equations (2) to (18) below:
 (1)
(1)
where:
mchar is the particle mass (kg)
t is time (s)
DH is the reaction heat (J.kgC-1)
2.1 The reaction heat DH
The reaction heat DH depends on the products of the reaction, which can be either CO or CO2 or a mixture of the two, according to the molar relationship shown in equation (2).
Char + ( 1 – fr/2 ) O2 -> fr CO + (1-fr) CO2 (2)
where fr is defined in equation (5) below.
The mass fraction CO/CO2 produced during the oxidation, rmass , can be estimated from
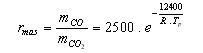 (3)
(3)
where
R is the [GLOSS]universal gas constant[/GLOSS] = 8.314 J.mole-1.K-1
Tp is the particle temperature (K)
The heat released by the char reaction can be rewritten in terms of the parameter fr and the reaction heats for the formation of CO and CO2 from the carbon in the char. The relationship is shown in equation 4:
 (4)
(4)
with 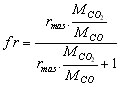 (5)
(5)
where
MCO2 is the molar mass of CO2 (kg/mole)
MCO is the molar mass of CO (kg/mole)
2.2 The carbon reaction rate
The carbon reaction rate is expressed as:
 (6)
(6)
in which the intrinsic reactivity kchar is expressed (kg.s-1.m-2.atm-1)
 (7)
(7)
where
SP is the specific surface area of the char (m2/kg)
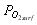 is the partial pressure of O2 at the particle surface (atm)
is the partial pressure of O2 at the particle surface (atm)
h is the effectiveness factor (-); see eq. (8)
Achar is the [GLOSS]frequency factor[/GLOSS] for the char oxidation reaction (kg.s-1.m-2.atm-1)
Echar is the [GLOSS]Activation energy[/GLOSS] for the char oxidation reaction (J/mole).
The effectiveness factor h is the ratio of the effectively reactive surface to the total pore surface of the particle. It accounts for any limitation of oxygen diffusion inside the particle. For a spherical particle, and first order kinetics, we have
 (8)
(8)
It is calculated from the [GLOSS]Thiele Modulus[/GLOSS] f
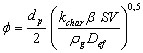 (9)
(9)
where
dp is the diameter of the particle (m)
rg is the density of the gas (kg/m3)
SV is the volumetric surface of the char (m2/m3) SV = rp . SP
b is the mass stoichiometric coefficient (kgO2/kgC)
The [GLOSS]effective diffusivity[/GLOSS] inside the particle Def can be expressed as follows, if one takes into account both molecular diffusion and [GLOSS]Knudsen diffusion[/GLOSS] in the smallest pores
 (10)
(10)
where
 is the porosity of the particle (-)
is the porosity of the particle (-)
t is the [GLOSS]tortuosity[/GLOSS] of the particle (-)
D is the [GLOSS]diffusivity[/GLOSS] of O2 in N2 (m2/s)
with the Knudsen diffusivity
 (11)
(11)
where MO2 is the molar mass of O2 (kg/mole)
The average pore diameter dpore (m) can be expressed as
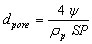 (12)
(12)
where rp is the particle density (kg/m3)
and the porosity is given by
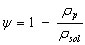 (13)
(13)
where rsol is the density of the solid fraction, or non porous carbon (kg/m3).
The oxygen flux F (mole 02/s) that is consumed by the reaction can be calculated from the carbon reaction rate, following the molar equation
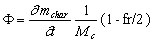 (14)
(14)
where MC is the molar mass of carbon (kg/mole).
If this flux is transferred by convection, we can also write
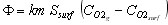 (15)
(15)
where
km is the O2 mass transfer coefficient at the surface of the particle (m/s)
Ssurf is the particle outside surface area (m2)
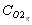 is the molar concentration of O2 in the gas phase (mole/m3)
is the molar concentration of O2 in the gas phase (mole/m3)
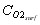 is the molar concentration of O2 at the surface of the particle (mole/m3)
is the molar concentration of O2 at the surface of the particle (mole/m3)
which enables us to calculate the oxygen concentration at the surface of the particle 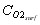 , and to derive the oxygen partial pressure in
, and to derive the oxygen partial pressure in 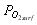 eq. (6). The calculation describes oxygen transfer process outside the particle.
eq. (6). The calculation describes oxygen transfer process outside the particle.
We also have
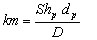 (16)
(16)
where the particle [GLOSS]Sherwood number[/GLOSS]Shp is equal to the particle Nusselt number Nup (see also Section 3 below)
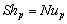 (17)
(17)NB: since the calculation of h and of F require the value for the reaction rate, and the calculation of the reaction rate requires the knowledge of h and F, the previous calculations need an iterative procedure to converge to the solution.
3. Heat exchanged with the surroundings: Qloss[W]
Taking into account the conductive/convective heat transfer and the radiative heat transfer, the heat flux exchanged between a spherical particle and its surroundings Qloss (W) can be expressed

(18)
where
h is the [GLOSS]convective heat transfer coefficient[/GLOSS] at the particle surface (W.m-2.K-1)
Tg is the gas temperature (K)
Tp is the particle temperature (K)
Tw is the furnace wall temperature (K)
e is the particle surface emissivity (-)
sO is the [GLOSS]Stephan-Boltzman constant[/GLOSS] (5.67.10-8 W.m-2. K-4 )
The particle surface convective heat transfer coefficient h can be calculated from
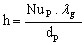 (19)
(19)where lg is the gas thermal conductivity (W.m-1.K-1)
The particle [GLOSS]Nusselt number[/GLOSS]Nup depends on the aerodynamic conditions. If the particle is small and thus being conveyed in the gas stream without slip, Nup = 2. If there is slip between the particle and gas, then
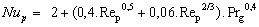 (20)
(20)where Prg is the gas [GLOSS]Prandtl Number[/GLOSS] (dimensionless).
The particle [GLOSS]Reynolds Number[/GLOSS]Rep is expressed as
 (21)
(21)
where mg is the gas viscosity (dynamic ) (kg.m-1.s-1).
The slip velocity between the particle and the gas Vslip can be expressed as follows, in the case where the particle is submitted to gravity
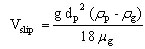 (22)
(22)
where rg is the gas density (kg/m3).
NOMENCLATURE
A frequency factor for carbon (char) oxidation kg.s-1.m-2.atm-1
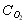 O2 [GLOSS]molar concentration[/GLOSS] of the gas mole/m3
O2 [GLOSS]molar concentration[/GLOSS] of the gas mole/m3
d diameter m
D diffusivity of O2 in N2 m2/s
E activation energy J/mole
h convective heat transfer coefficient W.m-2.K-1
k [GLOSS]intrinsic reactivity[/GLOSS] of carbon kgC.s-1.m-2.atm-1
km mass transfer coefficient m/s
m mass kg
M molar mass kg/mole
P pressure atm
Pr Prandtl number –
Re Reynolds number –
Sh Sherwood number –
SP specific surface area of the char m2/kg
SV volumetric surface of the char m2/m3
(SV = rp . SP)
T temperature K
b mass stoichiometric coefficient kgO2/kgC
r density kg/m3
 porosity of the particle (-)
porosity of the particle (-)
t tortuosity of the particle –
e particle surface emissivity –
l thermal conductivity W.m-1.K-1
m viscosity (dynamic) of the gas kg.m-1.s-1
h effectiveness factor –
f Thiele modulus –
s0 Stephan Boltzman constant = 5.67.10-8 W.m-2.K-4
DH reaction heat J.kgC-1
Constants
g gravity 9.81 m.s-2
R universal gas constant = 8.314 J.mole-1.K-1
Subscripts
char char, or carbon ( C )
ext surface of the particle
ef effective, inside the particle
g gas
p particle
pores pores
sol solid fraction
surf particle outside surface
w furnace walls
