-
What technologies are available to control mercury emissions from coal combustion?
Date posted:
-
-
-
Post Author
Patrick LaveryCombustion Industry News Editor
-
1. Background
Background information for mercury emissions can be found in CF59: What are the essential problems relating to mercury emissions from coal combustion? In brief, mercury emissions from coal combustion are difficult to control due to the volatility of mercury (resulting in high proportions of the total mercury being present as gas phase elemental mercury), the large volume of the [GLOSS]flue gases[/GLOSS], with high velocities, and the relatively low concentrations of mercury within them.
Release of mercury from fuel sources begins at ~150 oC, and is complete at temperatures of 500-600 oC. Elemental gaseous mercury is oxidised at temperatures between 300-400oC in the presence of HCl, Cl2, and also NO2. The boiling point of mercury is 357oC.
Legislation/Regulations/Guideline Limits
There are no existing limits for mercury emissions from power plants within the EU or US, though the US are planning to release draft guidelines in 2003 and final guidelines in 2004, and the EU is expected also to create guidelines in the future. The US guidelines would come into force in 2007.
Legislation will be the driving force for mercury control from [GLOSS]coal[/GLOSS][GLOSS]combustion[/GLOSS], as there is negligible damage to process equipment from mercury species.
2. Existing technologies that help reduce mercury emissions
Coal Cleaning
Conventional (physical) coal cleaning can result in around 37% decreases in mercury emissions from power plants, and advanced cleaning techniques can reduce emissions by up to 60%.
Particulate Control Devices
[GLOSS]Particulate control devices[/GLOSS] can reduce the total amount of mercury by trapping particle bound mercury. Typically, [GLOSS]ESP[/GLOSS]s remove around 32% and [GLOSS]fabric filter[/GLOSS] 44% of total mercury. The increased efficiency of fabric filters as compared with ESPs is due to the caking of carbon on the filters helping to reduce the mercury.
Flue Gas Desulphurisation (FGD)
Wet [GLOSS]FGD[/GLOSS]s on average remove 34% of total mercury, and dry scrubbers 30% of the emissions further. The difference may be due to the fact that wet FGDs may condense some of the gaseous elemental mercury.
3. Mercury specific technology
The only accepted technology to control mercury emissions from coal combustion is the injection of activated carbon, or the use of activated carbon in fixed beds. This can reduce total mercury emissions by up to 90%, but is expensive due to the very low concentrations of mercury in coal combustion flue gases. The mercury becomes attached to the activated carbon particle, either by physical adsorption or chemical bonding, and can then be trapped by particulate control devices.
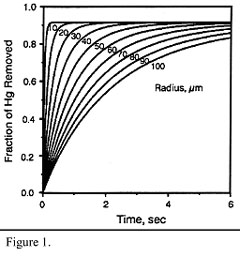
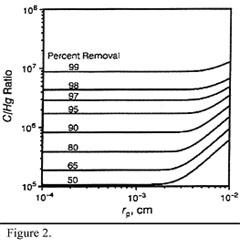
The C:Hg mass ratios required for effective removal are between 5000:1 and 100 000:1, depending on the temperature (removal increases at lower temperatures, indicating physisorption). Also, capture efficiency increases with decreasing particle size of the carbon (increased specific surface area). Figures 1 and 2 illustrate this. Around 1-2 seconds contact time is needed between the carbon and the flue gas. Impregnating the activated carbon with sulphur, iodine or chlorine increases the removal of mercury.
|
Figure 1: Removal of mercury (inlet: 20 mg/m3) with activated carbon at 140oC: effect of particle size and residence time (adapted from Brown et al., 1999) |
Figure 2: Removal of mercury (inlet: 20 mg/m3) with activated carbon at 140oC: effect of particle size and C/Hg mass ratio (adapted from Brown et al., 1999) |
There are options in the placement of the carbon injection point: upstream of the dust control devices, or downstream of them, with additional dust removal devices afterwards. Spray cooling can be implemented also to increase efficiency by encouraging condensation of the gaseous mercury.
Table 1 illustrates the approximated cost effectiveness of different types of activated carbon based mercury removal systems to remove >90% of total source mercury.
|
Removal System |
Flue Gas Temp (oC) |
Carbon Usage (g/g Hg) |
Total Capital Cost (1996 $US once off) |
O & M costs (1996 $US/yr) |
Cost Effectiveness (1996 $US /kg Hg) |
|
Direct injection of AC before particulate control device |
157 |
100000 |
$6.139 mil |
$30.411 mil |
$149 000 |
|
Particulate control, then spray cooling, then AC direct injection, then fabric filter |
93 |
9398 |
$41.62 mil |
$7.714 mil |
$56 000 |
|
Spray cooling after air preheater, then AC direct injection, then particulate control |
93 |
30000 |
$7.756 mil |
$11.404 mil |
$58 400 |
Table 1. Cost effectiveness of various activated carbon (AC) Hg removal systems for a coal fired power plant of boiler size 975Mwe, raw fluge gas Hg conc 10mg/[GLOSS]dscm[/GLOSS], removal 90%. Adapted from Brown et al (2000).
Activated carbon also adsorbs compounds such as SOx, NOx, HCl, H2O and dioxins and furans. The adsorption of HCl increases the removal of Hg (by oxidizing the elemental gaseous Hg), but SOx and NOx compounds decrease the Hg removal efficiency. Thus, the process requires a high degree of optimisation.
4. Future/Developing Technologies
As mercury is likely to be a more important issue in the future, as legislation is tabled and implemented, research is attempting to develop new technologies for mercury control.
Various metal compounds have been tested for their ability to capture mercury. Oxides such as MnO2, Cr2O3 and MoS2 have displayed moderate abilities, making them possible alternatives to activated carbon. Also, noble metal sorbents, especially gold, can be used to form an alloy with mercury, capturing up to 95% of gaseous mercury, regardless of form. The gold and mercury can be separated, the gold reused and the mercury sold. However, this technology has only had lab testing.
Other investigations aim to increase the efficiency of existing technologies, for example wet FGDs, carbon injection and ESPs.
Sources
[1] Zevenhoven, R. and Kilpinen, P., Control of Pollutants in Flue Gases and Fuel Gases, Helsinki, 2001
[2] US DOE, Mercury Control Technologies, http://www.fe.doe.gov/coal_power/existingplants/mercurycontrol_fs.shtml, 2002. Accessed June 2002.
[3] Brown, T.D. et al., Control of Mercury emissions from coal-fired power plants: a preliminary cost assessment and the next steps for accurately assessing control costs, Fuel Processing Technology, 2000.
[4] Brown, T.D. et al., Mercury measurement and its control: what we know, have learned and need to further investigate, Journal of the Air & Waste Management Association, 1999.