-
Update on IFRF-supported PhD project investigating the use of biological enzymes for the upgrading of CO2 to methanol
Date posted:
-
-
-
Post Author
Greg Kelsall
-
As part of a PhD project within the EPSRC Centre for Doctoral Training (CDT) in Resilient Decarbonised Fuel Energy Systems, supported by the IFRF, researcher Jennifer Hancock at the University of Sheffield is investigating the use of biological enzymes for the upgrading of CO2 to methanol. Now that she is more than half way through her studies, I asked Jennifer to give us a progress update, which she has kindly provided below:
It has become a widely accepted scientific fact that our conventional source of energy – the combustion of hydrocarbon fuels – is leading to dangerously high levels of atmospheric greenhouse gas (GHG) concentrations in the atmosphere. Carbon capture and conversion (CCC) could help to decarbonise a portion of ‘hard-to-abate’ sectors such as transport, heavy industry and smaller industries unable to qualify for carbon capture and storage (CCS), by capturing CO2 exhaust gases and transforming them back into in-demand fuels or chemicals used in other processes. Unfortunately, the inert nature and stability of CO2 presents a challenge to its efficient conversion. Large amounts of energy are required to break the highly stable bonds between the atoms, but once this barrier has been overcome, CO2 can serve as a highly flexible feedstock capable of producing over 150 different chemicals. Many of these are fine chemicals with niche markets; however more widely applicable fuels such as methanol (CH3OH) can also be produced.
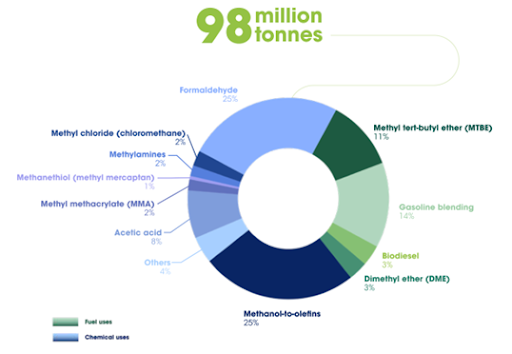
This ‘fuels and feedstock chemicals’ branch of CCC has attracted a lot of attention in recent years and has already been demonstrated in numerous studies and large-scale commercialised projects. Methanol [GK1] is a versatile and useful chemical for transportation fuels and as a foundation feedstock for thousands of other specialised chemicals. It is also the second most effective chemical, after urea, for sequestering anthropogenic CO2 emissions, with a capture capacity range between 1-10% of total annual emissions. Global production of methanol, currently around 98 Mt/year, is predominantly facilitated using coal (35%) and gas (65%) as the feedstock, with only 0.2 Mt produced renewably. This is contributing to rising direct CO2 emissions – from 183 Mt CO2 in 2015 to 211 Mt CO2 in 2018.
The simplicity of methanol as a hydrocarbon and its chemical energy has allowed it to become an established and valued chemical within our fossil-fuel centric system. As of 2017, methanol accounted for 19.6% of the global market’s production capacity for petrochemicals and contributed to the manufacture of over 90% of all organic chemical products. Methanol is also favoured due to the ease with which it can be transported and stored. Increasing use of methanol for both established and emerging applications, such as a source of hydrogen (H2) for flex-fuel and fuel-cell vehicles and for the creation of dimethyl ether – a super clean diesel fuel for transport means that demand is also expected to rise by over 50% by 2030. If methanol could be manufactured using waste CO2 from industries struggling to decarbonise and from smaller industrial units that do not qualify for CCS, the economic return of a valuable product might incentivise the substantial investment needed for carbon capture utilisation and storage (CCUS). It may also flip the production of methanol from a net-positive industrial activity to a net-neutral one, potentially preventing emissions of >211Mt CO2/year.
Many novel approaches have been proposed for CO2 to methanol conversion. These include CO2 conversion using renewable energy – particularly wind, solar and biomass, photoreduction using metal catalysts and biofixation using algae. Integrated methanol production systems have also been proposed alongside enhanced gas recovery (EGR) and dimethyl ether generation. Unfortunately, each of these methods has been met with challenges. In the case of photoreduction, rare-metal catalysts are often employed to drive the reaction in the desired direction, which have sustainability concerns. Biofixation has encountered issues with scaling-up production, with large outdoor reactors having been found to be less efficient for algal metabolic activity as those used in a laboratory setting. Additionally, significant energy and environmental costs have been associated with algal biomass extraction and processing. Whilst integrated methanol-EGR systems and DME generation have potential, the former is isolated to circumstances (geographical and economic) where CCS is also an option, and the latter requires high temperatures (250°C – 300°C) to drive the reaction in the desired direction.
In recent years, enzymes have become increasingly recognised as a powerful and sustainable alternative to inorganic catalysis for the conversion of CO2 to methanol. This is due to their ability to boost the activation energy required to reduce CO2 as well as their high substrate specificity, resulting in a faster reaction. Whilst enzymatic reduction of CO2 to methanol does not ordinarily occur under standard conditions, it is made possible by optimising the reaction conditions via a multi-enzymatic cascade system. Each works in reverse of the naturally occurring reaction, to reduce CO2 to formate via formate dehydrogenase, followed by reduction to formaldehyde via formaldehyde dehydrogenase and then finally to methanol, catalysed by alcohol dehydrogenase.
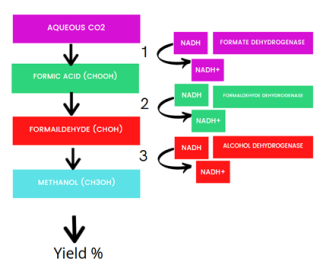
As part of her project, Jennifer is investigating the use of biological enzymes for the upgrading of CO2 to methanol. Despite the proven feasibility of the system from previous studies, this alternative system still presents a different set of considerations. Principally, a successful cascade system requires enzymes which have both high stability and activity to work within the higher temperatures and lower pH found in flue gas outflows from industrial sites. The use of free enzymes in solution also creates unfeasibly high operational costs due to low recovery rates. To overcome these challenges, she is exploring the possibility of enzyme immobilisation on biochar and bioinspired silica frameworks.
Biochar possesses unique chemical and physical features that could serve as a durable and cost-effective matrix for immobilised enzymes. A large surface area, facilitated by the presence of pores, creates a matrix-like structure within the biochar and should allow for high levels of enzymes to occupy the reaction-surface, leading to greater system efficiency. Pore size is important, since they should be large enough to allow an enzyme access beyond the surface of the support. Diffusion and mass transfer are also influenced by pore size and so should be considered. Following surface area analysis, it was determined that whilst a large biochar surface area may indeed be present in many biochars, practically it was unlikely to be the most effective framework due to the diameter and volume of the pores being unsuitable for enzyme functionality.
Whilst many of the chars studied did attain a suitable surface area (>25m2/g), none met the minimum pore diameter of 20-40nm recommended for immobilised enzyme catalysis systems, regardless of the activation method attempted. This suggests that whilst biochar may well have a large surface area, it is unlikely that enzymes will be able to operate efficiently since biochar does not meet the lowest pore diameter threshold required for an immobilisation matrix. Since scale up of industrial application for enzyme-mediated catalysis is primarily cost driven, it seems paramount to select the correct physical properties of the biochar to ensure maximum immobilisation efficiency and enzyme activity.
In light of this, the study focus has now shifted from biochar to investigating the feasibility of using silica as a catalytic framework instead. Silica is also able to form a highly ordered, chemically inert and porous substrate, and thus has also become a focus of investigation for researchers. Santa Barbara amorphous (SBA) silicas are amongst the most reported species as an immobilisation framework for carbonic anhydrase, a key enzyme under investigation for a myriad of different carbon-capture solutions.
