-
IFRF Summary of Research – Novel and High Performing Gas Diffusion Layers for Polymer Electrolyte Membrane Fuel Cells
Date posted:
-
-
-
Post Author
Greg Kelsall
-
As part of a PhD project within the EPSRC Centre for Doctoral Training (CDT) in Resilient Decarbonised Fuel Energy Systems, supported by the IFRF, researcher Fernando Ruscillo at the University of Sheffield is investigating the use of novel and high performing gas diffusion layers for Polymer Electrolyte Membrane fuel cells (PEMFCs). This is the second of three PhD projects supported by IFRF at the CDT, with the first covered in an earlier IFRF blog. Now that Fernando is more than half way through his studies, I asked him to give a progress update, which is provided below.
In the quest to adopt renewable energy technologies, many countries have been striving to transition various processes to use clean energy sources. However, the challenges in decarbonisation of specific sectors is very difficult due to the wide-ranging applications of fossil fuels.
One promising alternative to fossil fuels is the emergence of “green” hydrogen. This can be produced through the process of water electrolysis – a process which splits water into hydrogen and oxygen using electricity. If the electricity provided for electrolysis comes entirely from renewable sources, this process emits no greenhouse gases. Hydrogen presents several advantages, including: a high mass energy density, lightweight and ease of electrochemical conversion. These qualities make it a viable choice for conveying energy across extensive distances, either via pipelines, or shipped/trucked as a compressed gas or as a liquid (cryogenic or in the form of ammonia).
The growth of renewable energy usage in the wider energy landscape has provided a unique challenge in the form of intermittency. Solar and wind energy only produce electricity when the sun is shining or the wind is blowing. This poses a challenge to conventional grid designs which require stable energy sources.
One way to mitigate this is to develop energy storage technologies, such as: pumped hydro, compressed air energy storage, lithium-ion batteries, and hydrogen. Hydrogen, sourced sustainably from water, holds the potential for a near limitless supply of fuel and carbon free production. It also serves as an effective medium for energy storage, overcoming some of the limitations of current electricity storage technologies.
While hydrogen exhibits advantages, such as long-term storage capability and efficient conversion through fuel cells, it faces challenges like low volumetric energy density and the energy intensive process of converting it into a compressed gas or cryogenic liquid form. Additionally, hydrogen is not naturally abundant in an isolated state, instead being found in chemical compounds with other elements.
Fuel cells are positioned as a key technology in the future hydrogen economy, providing a cleaner and more efficient energy conversion method compared to other technologies, such as: internal combustion engines, gas turbines, coal-fired boilers, and steam turbines. Figure 1 below shows a schematic of the hydrogen economy and how fuel cell technology could be important for its development.
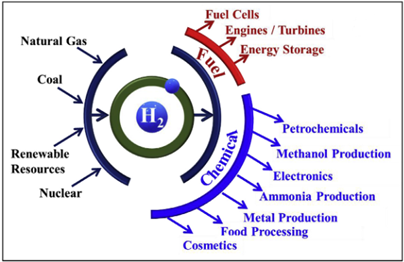
Figure 1 Hydrogen Production and Use Case Pathways Featuring Fuel Cells
Fuel cells are electrochemical devices that convert the chemical energy of the fuel and oxidising agent (hydrogen and oxygen), directly into electricity through an electrochemical reaction. Unlike a battery, which will run out of charge once the chemical constituents are fully converted. Fuel cells can undergo continuous operation if a steady supply of fuel is provided. Fuel cells offer advantages such as high efficiency, low emissions, and versatility in applications, ranging from small-scale portable devices to larger stationary power systems.

Figure 2 Components of a PEM Fuel Cell
The main components of a PEM fuel cell (shown in Figure 2) include the following:
- Bipolar plates (BPP)
- The BPP are fitted with flow channels that help distribute the reactant gases and they also act as current collectors in the fuel cell. They give the fuel cell structural support.
- Catalyst layer (CL)
- The catalyst enables the electrochemical reaction to take place by providing reactive sites for the gases. Platinum is typically used.
- Membrane
- The membrane is a thin layer of solid polymer electrolyte, between 10 – 100 μm. Its main purpose is to conduct hydrogen ions from the anode to cathode.
- Gas diffusion layer (GDL)
- GDLs play a critical role in a fuel cell. They distribute the reactant gases to the CLs, conduct electrons to the BPP, keep the membrane hydrated, transport excess liquid water away from the membrane and provide structural support to the membrane electrode assembly.
The research for this PhD project involves the characterisation and optimisation of the gas diffusion layer. The GDL can be subject to contact resistances within the fuel cell. This arises when different components come into contact with one another, giving rise to a non-continuous boundary which makes it difficult for electrons to pass through. This in turn increases the ohmic resistance and decreases the efficacy of the fuel cell. This is particularly important with regards to the GDL, as it sits in between the BPP and CL.
A microporous layer (MPL) is typically applied to the surface of the GDL which helps to improve its overall performance. An MPL comprises carbon black particles and PTFE. Conventionally, an MPL is applied to the side of the GDL facing the catalyst layer. This has been shown to improve contact resistance and water management within the fuel cell. The novelty of this research project is the application of a double sided MPL coated GDL, with a second thinner layer of MPL facing the BPP (Figure 3). This has been shown to increase the fuel cell performance by further lowering the contact resistance. However, there is a trade off between the thickness of the layers and the performance of the fuel cell.
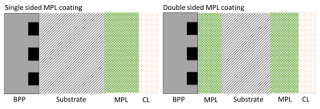
Figure 3 Configuration of a conventional single sided MPL coating and a novel double sided MPL coating
In order to fully understand this novel architecture, in depth characterisations were carried out, on both the single sided and double sided configuration. These characterisations included both ex-situ and in-situ testing.
- Ex-situ
- Permeability
- In-plane electrical conductivity
- Pore size distribution
- Hydrophobicity
- Morphology
- In-situ
- Polarisation curves
- Electrochemical impedance spectroscopy
The initial study looked at the characterisation of the single sided MPL coated GDL and the double sided MPL coated GDL, using two different types of carbon black for the MPL (Ketjenblack and Vulcan black). It was found that the double sided MPL coated GDL made from Vulcan black performed the best and improved overall fuel cell performance. Additional research is underway to further enhance this configuration. Firstly, by using novel materials in the MPL such as graphene nanoplates; and secondly, by modifying the microarchitecture of the MPLs by introducing pore forming agents.
