-
What are the essential NO reduction reactions used by in-furnace NOx control?
Date posted:
-
-
Post Author
dev@edge.studio
1.Overview
This Combustion File describes the essential NOx reduction reactions used for in-furnace NOx control. The techniques handled here are [GLOSS]reburning[/GLOSS] and [GLOSS]SNCR[/GLOSS]. Others, like [GLOSS]air staging[/GLOSS] are treated separately (CF128).
2. Reburning
Reburning is a combustion modification method that uses the fuel to reduce NOx emissions. About 10-20 % (energy) of the fuel – the reburn fuel – is added above the main combustion zone, to form a fuel-rich zone where NO is partially reduced to N2 and to other nitrogenous species (HCN, NH3). After the reburn zone, additional air is supplied to complete the combustion. In this burnout stage, the remaining fixed-nitrogen species (NO, HCN and NH3) are converted back to NO and/or to N2. Maximum reduction typically varies between 50-70 % or even higher (85%) if reburning is used together with ammonia or other additives.
Several fuels can be used as reburn fuel. Reburn, however, is most effective with methane ([GLOSS]natural gas[/GLOSS]). Reburn effectiveness is linked to the ability of fuels to readily produce hydrocarbon [GLOSS]radical[/GLOSS]s, which can rapidly react with NO, and is further enhanced if the reburn fuel does not contain any [GLOSS]organic fuel nitrogen[/GLOSS]. When the reburn fuel contains nitrogen, the NO reduction reactions include [GLOSS]NHi[/GLOSS] paths, which are explained below. Finally, reburning with non-hydrocarbon fuels like H2 and CO is also working for NO reduction, but with different reactions involved. All of them will be described in this CF.
2.1 Reburning with Hydrocarbon Fuels
2.1.1 Reburn zone
The chemistry in the reburn zone can be divided into two stages:
STAGE 1: Where the reburn fuel is oxidized to CO, H2 and H2O, under fuel rich conditions. The O2 concentration is depleted and high levels of radicals (H, OH, O and CHi) are produced. The general reaction paths for radicals formation are described as follows, e.g.,for the case with methane:
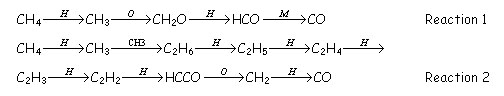
Hydrocarbon radicals’ production increases with the temperature.
Once hydrocarbon radicals have been formed, they react with NO to produce HCN. The most important reactions governing this conversion are:
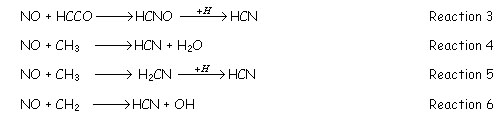
STAGE 2: during this period, there is low level of radicals and the main reactions occurring decompose the HCN previously formed to N2, NH3 and /or NO. These reactions are normally slower than the initial reduction of NO to HCN. The process is described as:
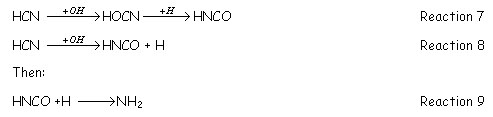
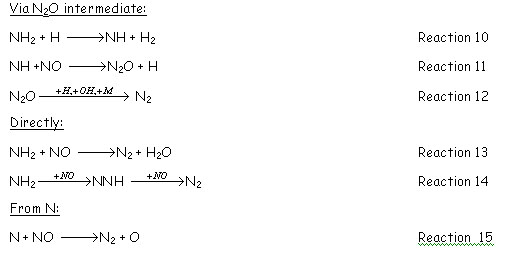
NH3 formation occurs via the reaction between NH2 and H2O, i.e., NH2+H2O=NH3+OH.
When the fuel contains [GLOSS]bound nitrogen[/GLOSS] (fuel-N see CF41), it adds many reactions to the reburning kinetics when comparing with hydrocarbon fuels. [GLOSS]Pyrolysis[/GLOSS] of nitrogen containing fuels forms HCN and NH3. Hydrogen cyanide follows the same sequence than the one described for reburning of hydrocarbon fuels. NH3, under reburning conditions (i.e. reducing atmosphere) reacts to form N2 following the general path:
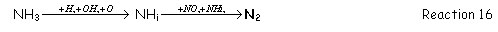
2.1.2 Burn-out zone
In this zone the remaining fixed nitrogen (NO, HCN and NH3) reacts to form N2 through different reaction paths, where the most important one is:
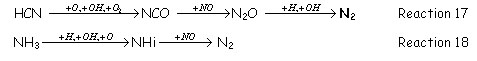
2.2 Reburning with non-Hydrocarbon Fuels: H2 CO.
H2 and CO may remove 20-30% of the NO entering the reburn zone, when they are used as a reburn fuel. The effect is increased with increasing temperature and increasing reburn fuel fractions. At high temperatures and reburn fuel fractions of about 30 %, the reduction efficiency approaches that of HC gases.
Conversion of NO to N2 in absence of hydrocarbon radicals and reactive nitrogen species such as NH3 or HCN potentially occurs through the reaction sequence:

However it depends on different mechanism dependent on temperature and stoichiometry.
· In absence of oxygen and temperature range between 900-1500K, the main reactions governing the NOx reduction are comparatively slow and they are:
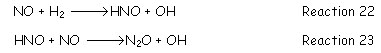
· In presence of oxygen it occurs via faster reactions involving radicals (reaction 19-20 and 21).
· Finally, at high temperatures, nitrogen oxide may be reduced directly by [GLOSS]endothermic reaction[/GLOSS] with hydrogen atoms.
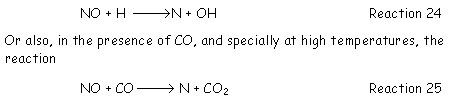
removes a significant fraction of NO.
3. Selective non-catalytic reduction
[GLOSS]Selective non-catalytic reduction[/GLOSS] is a process for NOx reduction that is usually applied to remove NOx from the exhaust gases, at the downstream end of the combustion process zone, or in the flue. It operates through the addition of a compound, which reacts with NO without the aid of a catalyst. SNCR works only within a specific temperature window.
SNCR reactions may be used in-furnace to improve reburning efficiency by enhancing the conversion to free nitrogen in the burnout zone. There are different SNCR rouutes depending on the additive selected to reduce NO, which can be ammonia principally, but also [GLOSS]urea[/GLOSS] or [GLOSS]cyanuric acid[/GLOSS].
3.1 Thermal De-NOx process
Thermal De-NOx process is a SNCR used in a lot of industrial applications. The additive used is ammonia (NH3). The ammonia decomposes to amine radicals (NHi) due to the presence of OH and oxygen atoms. Then NHi reacts with nitric oxide to form N2
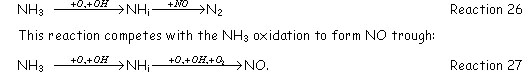
However the balance between reduction and oxidation is favorable when:
· The temperature ranges between 1100- 1250 The temperature range can be shifted to lower temperature by radicals produced by additives.
· The ammonia must mix uniformly into the hot flue gas in the presence of excess O2.
Ammonia injection is usually more effective when it is added in the form of a gas.
NO reduction with ammonia is successful only if the negative consequences are small. Thus, when ammonia and additives are injected, special attention is paid to the bypass of ammonia to the stack, to increments in the CO emissions, and to changes in the emission of nitrous oxide (N2O).
In general the injection of ammonia can contribute to significant NO reduction (50-70 %). For CFB boilers, this reduction leads down to a level in the order of 60-20 ppm.
3.2 NOx-OUT process
The [GLOSS]NOx-OUT process[/GLOSS] is a SNCR process where the additive used to reduce NO is urea. The global reaction governing the process is described as follows:
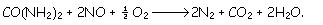
The optimum temperature window varies between 1150- 1300K.
The addition of urea may increase emission of N2O, which is its major drawback.
Acknowledgements
The author would like to thank Dr. Pia Kilpinen for her support, help as well as for the information given.
Sources
[1]Kilpinen P., Glarborg P. and Hupa M.: Reburning Chemistry: A Kinetic Modeling Study, Ind. Eng. Chem. Res., Vol. 31, No. 6 1992.
[2] Charles E. Baukal, J.R: The John Zink Combustion Handbook, New York,2001.
[3] P. Glarborg, P.G. Kristensen, K. Dam-Johansen, M.U. Alzueta,A. Millera, and R. Bilbao; “Nitric Oxide Reduction by Non-Hydrocarbon Fuels”, Energy and Fuels 14, 828-838, 2000.
