-
What is the gas-phase mechanism for NO formation from N2O and when is this important in combustion systems?
Date posted:
-
-
Post Author
dev@edge.studio
1. Background on NOx
[GLOSS]Nitrogen Oxides[/GLOSS] ([GLOSS]NOx[/GLOSS] = NO+NO2) are among the most severely regulated pollutants emitted from combustion systems. NOx are known to have adverse effects on human health and to cause environmental degradation (See CF125).
Four mechanisms for NOx formation are generally recognized – [GLOSS]Thermal NO[/GLOSS](CF40), [GLOSS]Prompt NO[/GLOSS](CF42), [GLOSS]N2O intermediate[/GLOSS] NOx, and [GLOSS]Fuel NO[/GLOSS] (CF41), (See CF66).
This Combustion File focuses on the N2O-intermediate mechanism.
2. N2O-intermediate NOx mechanism
The N2O-intermediate NOx mechanism was proposed first by Malte and Pratt (1974) for NO formation from molecular nitrogen (N2) via nitrous oxide (N2O). The N2 enters combustion systems mainly with combustion and dilution air. Under favourable circumstances, this mechanism may contribute to as much as 90% of the NOx formed in combustion (Chapter 3).
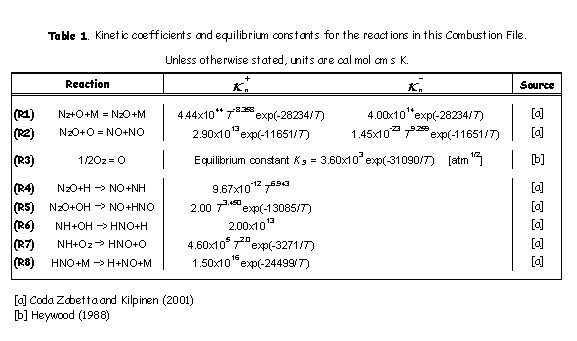
This mechanism takes into account two reversible elementary reactions:

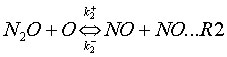
where k+ and k– are the rate coefficients for the forward and backward reaction respectively, and their expressions are given in Table 1.
M is a general third body. Because reaction (R1) involves third bodies (M), the mechanism is favoured at elevated pressures. Because both reactions (R1) and (R2) involve the oxygen radical (O), the mechanism is favoured at oxygen-rich conditions (l= 1/f>> 1).
According to the kinetic rate laws, the rate of NOx formation via the N2O-intermediate mechanism is:

where square brackets indicate molar concentrations (mol cm-3). Solving of (Eq.1) requires the knowledge of [O] and [N2O].
While not always justified, it is often assumed that the radical O originates solely from the dissociation of molecular oxygen (O2),
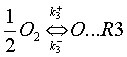
and that reaction (R3) is at equilibrium, which implies:
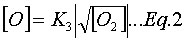
In addition, it is often assumed that the N2O is at quasi-steady-state (![]() ), which implies:
), which implies:
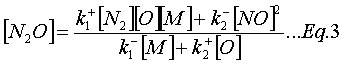
The system of equations (Eq.1-3) solves the rate of formation for NOx while knowing the concentration of species (N2, O2, and M), the kinetic rate coefficients of reactions (R1-2), and the equilibrium constant of (R3).
Coda, Zabetta and Kilpinen (2001) have added an extension to the N2O-intermediate mechanism, called N2O-extension mechanism. This mechanism adds to reactions (R1-2) also the following reactions:
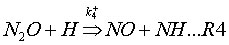
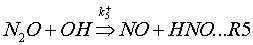
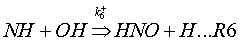


3. Combustion systems where the mechanism is important
The relevance of NOx formation in gas-phase from N2O has been observed indirectly, and theoretically speculated for a number of combustion systems and by a number of researchers: Barlow et al. (2000), Correa (1992), Glarborg et al. (1994), Steele et al. (1995), Tabacco et al. (2002).
The N2O-intermediate mechanism, as well as its extension, is favoured at elevated pressures and in oxygen-rich conditions. This makes it particularly important in highly pressurized devices operated with large excess of oxygen, i.e., gas turbines and compression ignition engines. As these devices are operated at increasingly low temperatures to prevent NOx formation via the thermal NOx mechanism (CF66), the relevance of the N2O-intermediate mechanism is increasing. It has been observed that over 30% of the NOx formed in these systems may be attributed to the N2O-intermediate mechanism.
The N2O-intermediate mechanism may be also of outstanding importance in systems operated in flameless mode (See CF171 and 174). These systems are referred to with many technical and commercial names: diluted combustion, [GLOSS]flameless combustion[/GLOSS], flameless oxidation, FLOXÒ, etc. In flameless mode, the fuel and oxygen are highly diluted in inert gases, so that the combustion reactions and the resulting heat release are carried out in a diffused zone. As a consequence, elevated peaks of temperature are avoided, which prevents thermal NOx. According to sources, the N2O-intermediate mechanism may contribute to about 90% of the NOx formed in flameless mode, and the remaining 10% should be attributed to the Prompt NOx mechanism (See CF66).
Sources
[1] Barlow R. S., Fiechtner G. J., Carter C. D., Chen J.-Y., Experiments on the Scalar Structure of Turbulent CO/H2/N2 Jet Flames, Combust. Flame, 120, 549-569, 2000
[2] Coda Zabetta E., Kilpinen P., Improved NOx Submodel for In-Cylinder CFD Simulation of Low- and Medium-Speed Compression Ignition Engines, Energy & Fuels, Vol. 15, No. 6, pp. 1425-1433, 2001
[3] Correa S. M., A Review of NOx Formation Under Gas-Turbine Combustion Conditions, Combust. Sci. and Tech., 87, 329-362, 1992
[4] Glarborg P., Johnsson J. E., Dam-Johansen K., Kinetics of Homogeneous Nitrous Oxide Decomposition, Combust. Flame, 99, 523-532, 1994
[5] Heywood J. B., Internal combustion engine fundamentals, Duffy, A., Morriss, J. M., Ed., McGraw-Hill, ISBN 0-07-028637-X, 1988
[6] Kilpinen P., Norström T., Mueller C., Kallio S., Hupa M., Homogeneous NO and N2O Chemistry in FBC, 38th IEA Fluidized Bed Conversion Meeting, Savannah, May 15-16, 1999
[7] Malte P. C., Pratt D. T., Measurement of Atomic Oxygen and Nitrogen Oxides in Jet-Stirred Combustion, in Proceedings of the 15th Symposium (International) on Combustion, pp. 1061-1070, The Combustion Institute, Tokyo, Japan, August 25-31, 1974
[8] Steele R. C., Malte P. C., Nicol D. G., Kramlich J. C., NOx and N2O in Lean-Premixed Jet-Stirred Flames, Combust. Flame, Vol. 100, pp. 440-449, 1995
[9] Tabacco D., Innarella C., Bruno C., Theoretical and Numerical Investigation on Flameless Combustion, to appear in Combust. Sci. and Tech., 2002
