-
What is coal ash?
Date posted:
-
-
Post Author
dev@edge.studio
1. Background
Industrial fuels are introduced in Combustion File (CF) 62 and more specifically [GLOSS]coal[/GLOSS] is introduced in CF177.
The present CF introduces coal [GLOSS]ash[/GLOSS] as an introduction to a portfolio of CFs examining coal ash in more detail, with a particular emphasis upon problems created by coal ash in combustion equipment such as pf fired boilers – [GLOSS]slagging[/GLOSS] and [GLOSS]fouling[/GLOSS] – and the disposal of [GLOSS]fly ash[/GLOSS]. For a detailed introduction, see references 1 and 2.
2. Ash forming species
There is no ash present in coal. However there are ash-forming species present, which during thermal conversion of coal are transformed into ash being present as fly ash, bottom ash or in deposits after the thermal conversion process.
Thus, ash is formed from inorganic, non-combustible material, which is either an integrated part of the fuel or introduced to the combustor with the fuel (such as dirt, clay etc.).
The content of inorganic constituents in coal are usually given as the oxides of the relevant metal. In a standard bulk chemical ash analysis, the composition is given as [2]:
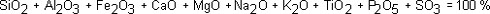
where SiO2 is the weight percentage of Si, expressed as SiO2 in the ash.
The coal ash composition varies over a large range, being strongly dependent on the rank as well as geographic origin of the coal, as seen in Table 1.
|
Oxide Component: |
Percentage: |
|
SiO2 |
10 – 70 |
|
Al2O3 |
8 – 38 |
|
Fe2O3 |
2 – 50 |
|
CaO |
0.5 – 30 |
|
MgO |
0.3 – 8 |
|
Na2O |
0.1 – 8 |
|
K2O |
0.1 – 3 |
|
TiO2 |
0.4 – 3.5 |
|
SO3 |
0.1 – 30 |
Table 1: Chemical composition ranges for coal ash.
3. Form of inorganic constituents
The inorganic constituents in coal can occur in several forms in amorphous phases [1]:
· As discrete minerals (mineral grains),
· As organically associated cations,
· As salts (cations) dissolved in pore water in the coal.
Figure 1 shows these forms of occurrence.
The mineral grains normally comprise a large part of the inorganics in coal. For the higher rank coals, [GLOSS]bituminous coal[/GLOSS] and [GLOSS]anthracite[/GLOSS], most of the inorganic species are present as minerals.
Minerals present in the coal matrix can be classified as included or excluded minerals. The included minerals are closely associated with the organic coal matrix, where excluded minerals are not.
A list of mineral species often found in coal and their chemical formula is in provided in Table 2.
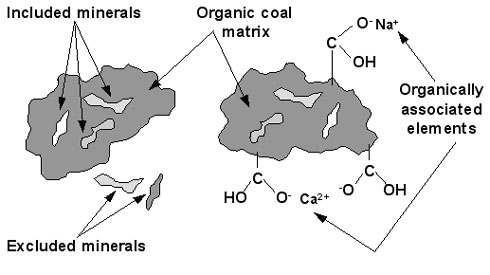
Figure 1: Illustration of modes of occurrence for minerals and
other inorganic constituents in coal [1].
Mineral |
Formula |
Mineral |
Formula |
Albite |
Na2O.Al2O3.6SiO2 |
Calcite |
CaCO3 |
Dolomite |
CaCO3.MgCO3 |
Gypsum |
CaSO4.2H2O |
Halite |
NaCl |
Hematite |
Fe2O3 |
Illite |
K2O.3Al2O3.6SiO2.2H2O |
Kaolinite |
Al2O3.2SiO2.H2O |
Magnetite |
Fe3O4 |
Muscovite |
K2O.3Al2O3.6SiO2.2H2O |
Orthoclase |
K2O.Al2O3.6SiO2 |
Pyrite |
FeS2 |
Quartz |
SiO2 |
Rutile |
TiO2 |
Siderite |
FeCO3 |
Sylvite |
KCl |
Table 2: Name and chemical formula of some minerals commonly found in coal.
The fraction of organically associated (= organically bound = atomically dispersed) inorganic constituents varies with coal rank.
When the fuel rank decreases (H:C and O:C ratios increase), the fraction of organically associated inorganic components increase.
Approximately 25% of the oxygen is present as carboxylic acid groups, which can act as bonding sites for cations such as Na+, K+, Ca2+ etc [1-2].
Another association form are the [GLOSS]chelates[/GLOSS], where the cations form a chelate coordination complex with pairs of adjacent organic oxygen functional groups.
4. Conclusions
This foregoing competes the answer to the basic question “what is ash?”.
The subject is pursued further with CF39 and CF77, dealing respectively with factors affecting coal ash formation and the analysis of coal ash chemistry.
Acknowledgements
CHEC is co-funded by ELSAM, ENERGY E2, the Danish Technical Research Council, the Danish and Nordic Energy Research Programmes, and the European Union.
Sources
[1]Benson, S.A., Jones, M.L. and Harb, J.N., 1993, Ash formation and deposition. In Smoot, L.D. (Ed), Fundamentals of Coal Combustion – for Clean and Efficient Use, Coal Science and Technology 20, Elsevier Science Publishers, Amsterdam, ISBN 0-444-89643-0, Chapter 4, pp. 299-373
[2] Bryers, R.W., 1996, Fireside slagging, fouling and high temperature corrosion of heat-transfer surface due to impurities in steam-raising fuels, Prog. Energy Combust. Science, 22 (1), pp. 29-120
