-
What factors affect coal ash formation?
Date posted:
-
-
Post Author
dev@edge.studio
1. Background
This Combustion file (CF) stems from CF38 What is coal ash? The reader is also referred to CF177 for an introduction to [GLOSS]coal[/GLOSS].
2. Mechanisms of Coal Ash Formation
Coal ash is formed by two major mechanisms, each of which leads to a peak in the, at least, bimodal size distribution of [GLOSS]fly ash[/GLOSS], as shown in Figure 1.

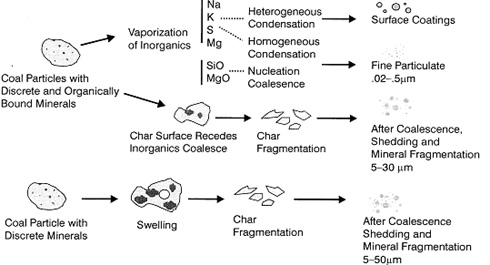
Figure 1: Inorganic metal transformations during coal combustion [1].
The major peak from 3-50 mm comprises the so-called [GLOSS]residual ash[/GLOSS], and has a mean particle size of about 10 mm. The residual ash constitutes approximately 98-99 % (w/w) of the total ash.
The minor peak comprises the sub-micron ash particles, and has a mean particle size of about 0.1 mm. The sub-micron ash particles constitute approximately 1-2 % (w/w) of the total ash, and is often named [GLOSS]inorganic fume[/GLOSS].
The distribution between residual ash and submicron ash, and the mean size of those two ash fractions, is related to the [GLOSS]coal rank[/GLOSS] fired and the design of the coal grinding equipment. A more detailed illustration of the ash formation mechanisms for residual and sub-micron ash are given in Figure 2.
During the combustion of a [GLOSS]char[/GLOSS] particle, the mineral inclusions in the particle will approach each other, forming ash agglomerates. Due to the combustion of the char, the temperature is so high that the ash can melt and form large spherical droplets on the surface of the burning char particle.
However, during combustion, the char particles usually fragment due to internal formation of gases or thermal shock, and thereby limit the degree of agglomeration of ash, forming a number of ash particles, from each coal particle, depending on the rank of the coal. On average, 3-5 ash particles are formed from each coal particle.
|
|
Mineral grains can also fragment during heating, and thereby limit the degree of coalescence. However, this is mainly of importance for reactive and excluded minerals, like pyrite and calcite. The variation in composition of ash particles is due to the distribution of minerals in the coal particles.
Fly ash from high-rank coal firing in pulverised coal fired boilers consists mainly of silicate glass (i.e. a liquid with high viscosity), and can be considered to be 90-95% amorphous. When burning low-rank coals, salt and oxide phases like CaSO4, CaO and MgO are common in the fly ash.
Sub-micron ash particles are formed from flame-volatilised inorganic constituents in the coal, or due to thermal or chemical decomposition (inorganic reaction) of the inorganic constituents.
During the char burnout, a large degree of the heat release takes place at the char particle surface where carbon is oxidised to CO. The reducing atmosphere thus created will be very hot, thereby providing the driving force for vaporisation of inorganic species.
In the near vicinity of the char particle surface, super-saturation of volatilised inorganics can occur, thereby leading to nucleation and the formation of very small particles. These can then grow by coagulation and homogeneous condensation of vaporised inorganics.
The particles may agglomerate and afterwards coalesce into spheres. Coalescence stops when the particles are cooled below the melting point of the material. The resulting particles can coagulate and form agglomerates, thereby leading to a narrow size distribution of the particles, between 0.01 mm and 0.1 mm.
The remainder of the volatilised inorganic species will condense on heat transfer surfaces or particles already formed and entrained in the flue gas. The surface composition of fly ash have been found to be enriched in alkaline and other volatile elements, thereby quite possibly increasing the deposition propensity of the ash.
3. Conclusions
Coal combustion produces a bimodal fly ash particle size distribution, where the residual ash – ie. the big ash particles – constitute by far the largest weight fraction (up to or even above 95 % (w/w)).
The sub-micron ash fraction is formed due to species that are volatilised in the flame and subsequently supersaturated or condensed heterogeneously on the surface of the residual fly ash particles.
The sub-micron peak constitute a potential emission risk, since a number of trace elements are known to condense on the surface of these particles, which are very difficult to retain in any sort of particulate removal device.
This gives rise to a development of a future knowledge base covering fine particulates. This is introduced by CF58: What are trace metal emissions?
Acknowledgements
CHEC is co-funded by ELSAM, ENERGY E2, the Danish Technical Research Council, the Danish and Nordic Energy Research Programmes, and the European Union.
Sources
[1]Benson, S.A., Jones, M.L. and Harb, J.N., 1993, Ash formation and deposition. In Smoot, L.D. (Ed), Fundamentals of Coal Combustion – for Clean and Efficient Use, Coal Science and Technology 20, Elsevier Science Publishers, Amsterdam, ISBN 0-444-89643-0, Chapter 4, pp. 299-373
[2] Bryers, R.W., 1996, Fireside slagging, fouling and high temperature corrosion of heat-transfer surface due to impurities in steam-raising fuels, Prog. Energy Combust. Science, 22 (1), pp. 29-120
[3] Flagan, R.C. and Friedlander, S.K., 1978, Particle Formation in Pulverized Coal Combustion – A Review, In D.T. Shaw (Eds.), Recent Developments in Aerosol Science. New York: John Wiley & Sons.
