-
What are the essential problems related to mercury emissions from coal combustion?
Date posted:
-
-
-
Post Author
Patrick LaveryCombustion Industry News Editor
-
1. Background
Mercury is classified as a [GLOSS]trace metal[/GLOSS], and is emitted from [GLOSS]coal[/GLOSS] [GLOSS]combustion[/GLOSS] systems both in the gas and particle phases (to a limited extent). Amongst trace metals, it has singular problems due to the difficulty in controlling emissions, as it is highly volatile. The boiling point of mercury is 3570C, while its melting point is –390C.
Mercury emissions can be as elemental mercury (gaseous or particle bound), or in an oxidised form (gaseous or particulate), such as HgCl2 (g, s), Hg2Cl2 (s), HgS (s, g), HgO (s, g) and HgSO4 (s, g). Once emitted, elemental mercury can react to form the [GLOSS]organic[/GLOSS] CH3Hg.
2. Emissions
Amount emitted
Coal-fired power plants are the largest contributor to man made mercury emissions (~65%wt of anthropogenic sources), and uncontrolled emissions are estimated as around 0.5kg/MWelec per year. Typically, mercury emissions from power plants are in concentrations of around 1-10mg/m3STP (0.8 – 8 ppbw) while the raw [GLOSS]flue gases[/GLOSS] before treatment have about 5-50 mg/m3STP (4 – 40 ppbw).
Sources
Mercury is present in trace quantities in most fuels, ranging from <0.01 mg/kg in [GLOSS]PET coke[/GLOSS] to up to 10 mg/kg-fuel in sewage sludge. Coal typically has between 0.02 and 3 mg/kg-fuel mercury, which is usually strongly associated with pyrite and other sulphites in coal. When the fuel is combusted, mercury is volatilised and is passed to the post combustion units of the process.
Health and Environmental Effects
Mercury is a highly toxic substance. [GLOSS]Acute[/GLOSS] effects of elemental and methyl mercury (an organic form formed when inorganic mercury enters aquatic systems) are to the central nervous system (for example hallucinations, blindness, deafness). [GLOSS]Inorganic[/GLOSS] mercury affects the [GLOSS]gastrointestinal[/GLOSS] and respiratory systems from acute exposure. [GLOSS]Chronic[/GLOSS] health effects are to the central nervous system and the kidneys. The US [GLOSS]EPA[/GLOSS] reference dose (maximum allowed daily dosage) for methyl mercury is 0.0003 mg/kg body weight/day. Both inorganic mercury (as a general group) and methyl mercury are listed as possible human carcinogens by the same organisation.
Oxidised Hg is deposited locally around the source of the emission, but elemental Hg can be transported several hundred kilometres, making mercury both a local and a global problem. Mercury in the environment is circulated through re-volatilisation, deposition and uptake/consumption.
Guideline Emission Limits
Currently, guidelines do not exist in the US or the EU for the emissions of mercury from coal-fired power plants. The US EPA announced in December 2000 that they will propose emission regulations for mercury in December 2003, and will issue final regulations in December 2004. It is expected that the EU will also issue regulations in the future.
Mercury is typically present in concentrations that do not correspond to damage to process equipment. The likelihood of stringent future legislation is the motivating factor for control.
3. Control of Mercury Emissions
Control in industries other than coal combustion
In industries such as [GLOSS]MSW[/GLOSS] incineration, where emission regulations exist, control of mercury emissions is through the use of technologies such as:
· Scrubbers and adsorbers (which remove oxidised mercury)
· Oxidation of elemental mercury by injection of gaseous [GLOSS]Na2S4[/GLOSS] or similar oxidising agent into the flue gas before the particulate control device
· Capture of the Hg by activated carbon
The use of these technologies can result in >90%wt removal of the total mercury present, which in many systems is enough. Control of mercury is dealt with in more detail in CF188 and CF189.
Control of mercury emissions from combustion is difficult. The reasons for this are:
· Flue gas streams are large, and mercury concentrations are low (in the order of 0.001ppmw). This creates mass transfer limitations.
· Flue gas velocities are high, and residence times are short. This creates kinetic limitations.
· Mercury is present in gas phase elemental form (insoluble in water), in particle bound elemental form, and in soluble compounds in both phases
· The ratio of Cl:Hg is low, which means there is less oxidised Hg, making control more difficult. At ~0.2% wt (800 ppmv) Cl in the flue gas, elemental mercury is <20%wt of the total mercury. However, when there is <0.1% wt (400ppmv) Cl, elemental mercury is >50%wt.
Essentially, the problems relating to mercury emissions from combustion boil down to the fact that mercury is difficult and expensive to control due to the high fraction (~90%) of the mercury being present in the flue gas, much of it in the elemental form (the most difficult to control). Of course, the underlying problem is that mercury is highly toxic. Figure 1 gives an indication of the fate of coal mercury, i.e. what proportion of the total source mercury 1) ends up in the elemental vapour phase, 2) in the oxidised vapour form, 3) in the bottom ash. The variation in this (one positive and one negative standard deviation each way) between coals is also shown by the error bars.
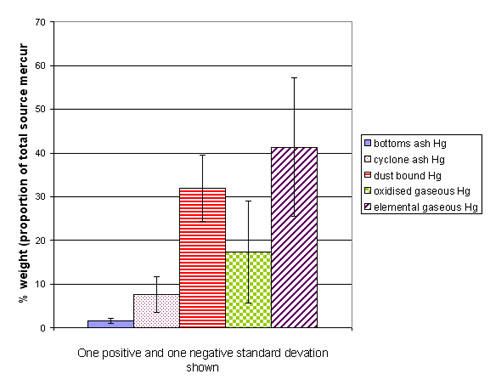
Figure 1. Fate of coal mercury, average of 3 coals fired in a lab scale combustor (adapted from Tsuji et al., 2000)
The traditional units used for flue gas clean up in coal combustion are usually not highly effective in removing mercury. Emerging/recent technologies may be more effective. Table 2 lists the efficiencies for removing mercury of typical coal combustion flue gas clean up units. It should be noted these are highly variable – in some cases, no Hg removal occurs over the [GLOSS]ESP[/GLOSS] or [GLOSS]fabric filter[/GLOSS].
|
Unit |
Particulate Control Scrubber |
ESP |
Fabric Filter Baghouse |
[GLOSS]FGD[/GLOSS] wet |
FGD dry |
|
Hg Removal Efficiency |
4 % |
32 % |
44 % |
34 % |
30 % |
Table 2. Efficiencies for removal of Hg from flue gases of typical coal combustion flue gas clean up units in the US (information from Zevenhoven and Kilpinen, 2001)
Mercury emissions for coal combustion are typically controlled by [GLOSS]activated carbon[/GLOSS] injection, but this is expensive due to the high carbon:mercury ratios required. Costs are estimated at between $US 56 000 and $US 149 000 /kg Hg removed for capital and operating costs (1996 US currency). Conventional coal cleaning can eliminate up to 40% of the mercury, though higher removal rates are possible with more advanced techniques. More detail for control of mercury from coal combustion can be found in CF188.
Sources
[1] Zevenhoven, R. and Kilpinen, P., Control of Pollutants in Flue Gases and Fuel Gases, Helsinki, 2001
[2] US EPA, Hazard Summary – Mercury Compounds, http://www.epa.gov/ttn/uatw/hlthef/mercury.html, 2001. Accessed June 2002.
[3] Tsuji, T. et al., A study on mercury behaviour on coal combustion using a thermo-balance and a drop-tube furnace and a bench-scale coal combustion furnace, Japan, 2000
[4] Swaine, D.J., Why trace elements are important, Fuel Processing and Technology, 2000
[5] Laudal, D.L. et al, Effects of flue gas constituents on mercury speciation, Fuel Processing Technology, 2000.
[6] Olson, E.S. et al, Catalytic effects of carbon sorbents for mercury capture, Journal of Hazardous Materials, 2000.
[7] Brown, T.D. et al., Control of Mercury emissions from coal-fired power plants: a preliminary cost assessment and the next steps for accurately assessing control costs, Fuel Processing Technology, 2000.
