-
What are the chromium manufacturing processes?
Date posted:
-
-
Post Author
dev@edge.studio
1. Introduction
The complete range of processes undertaken in the minerals processing industry was outlined in CF 255. This combustion file gives more specific detail of the processes in use for the manufacture of chromium.
2. Chromium
Chromite ore (FeCr2O4) is the only commercial source of chromium. Most of this ore is converted to sodium chromate (and dichromate) by roasting with sodium carbonate and [GLOSS]lime[/GLOSS]. The roasting process is carried out in a [GLOSS]rotary kiln[/GLOSS], and involves a number of temperature dependent reaction steps.
ambient to 450oC preheating of feed
~450oC oxidation of FeO to Fe2O3
450oC to 700oC bed heating
700oC to 900oC combination of alumina and soda ash to NaAlO2
900oC to 1100oC bed heating
~1150oC exothermic reaction of Cr2O3 with NaAlO2 and oxygen to form Na2CrO4 and Al2O3
The final reaction occurs in a [GLOSS]flux[/GLOSS], and even slight overheating of the bed results in the formation of an excessive burning zone coating and rings. Figure 2 shows the breakdown of world chromite ore production by major countries of origin. The processing of the ore to sodium chromate(s) is also carried out in the countries that are major users, thus there are roasting plants in North America, Japan, Europe and China.
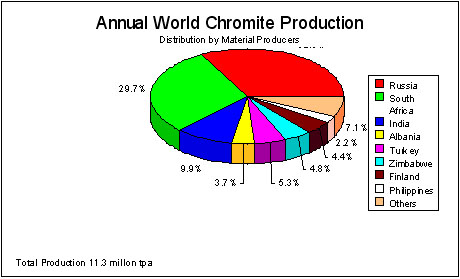
Figure 2 World Production of Chromite Ore in 1993 (The Economics of Chromium)
Sources
[1] European Minerals Yearbook
[2] Industrial Minerals Handy Book
[3] The Economics of Chromium
