-
What are the mechanisms for Prompt NOx formation during combustion?
Date posted:
-
-
Post Author
espadmin
Overview
There are basically four recognized mechanisms of NOx formation – [GLOSS]Thermal NO[/GLOSS],[GLOSS]Prompt NO[/GLOSS], [GLOSS]N2O intermediate[/GLOSS], and [GLOSS]Fuel NO[/GLOSS]. These are outlined in CF66. This CF focuses on the mechanisms for prompt NO formation.
Prompt NO, also called the [GLOSS]Fenimore NO[/GLOSS], is produced rapidly in the flame front, before there would be time to form NO by the thermal mechanism (see CF40).
Various arguments have been proposed to explain the rapid formation of NOx within the flame front. Some authors postulate that the observed NO formed in the flame front, is indeed produced by an enhanced effect of thermal NO caused by an overshoot of [GLOSS]radical[/GLOSS] concentrations (O, OH) above their thermodynamic equilibrium values.
A more plausible explanation is, however, provided by reactions involving hydrocarbon radicals and atmospheric nitrogen (N2), which form an intermediate compound, hydrogen cyanide (HCN), that is further oxidized to form NO.
2. Chemistry of Prompt NO formation.
2.1 Reaction description
Prompt NO formation involves three overall steps, which are described below. The corresponding rate coefficients are show in Table 1.
– Hydrocarbon radical formation
Occurs in the initial stages of the fuel pyrolysis and oxidation process of the fuel hydrocarbon compounds. The most important hydrocarbon radicals from prompt NO formation point of view are CH and CH2. The mechanism of CH and CH2 formation depends somewhat on the fuel but in most cases the most important ones are the following:
CH3 + H  CH2(S) + H2 Reaction (1)
CH2(S) + H2 Reaction (1)
CH3 + OH  CH2(S) + H2O Reaction (2)
CH2(S) + H2O Reaction (2)
CH2(S)+ M  CH2 + M Reaction (3)
CH2 + M Reaction (3)
The CH2(s) is the singlet species of methylene. In all cases CH comes from methylene by reaction with H and OH radical:
CH2+ H  CH + H2 Reaction (4)
CH + H2 Reaction (4)
CH2 + OH  CH + H2O Reaction (5)
CH + H2O Reaction (5)
– Atmospheric nitrogen fixation
Then, hydrocarbon radicals react with molecular nitrogen to form cyano-compounds (HCN/CN) and amines (NHi) . The two principal reactions involved are:
CH + N2  HCN + N Reaction (6)
HCN + N Reaction (6)
CH2 + N2  HCN + NH Reaction (7)
HCN + NH Reaction (7)
Where reaction 6 is the primary path and it is the rate-limiting step of the total prompt NO sequence.
– Interconversion between fixed nitrogen species
Once hydrogen cyanide (HCN) is formed, it leads to the formation of amine species (NH2, NH, and N) through different routes depending on the combustion environment.
· In fuel-lean atmospheres ([GLOSS]equivalence ratio[/GLOSS]s less than about 1.2), HCN reacts to form NH:
HCN + O  NCO + H Reaction (8)
NCO + H Reaction (8)
NCO + H  NH + CO Reaction (9)
NH + CO Reaction (9)
NH + H N + H2 Reaction (10)
N + H2 Reaction (10)
N + O2/ OH  NO + O/H Reaction (11)a/b
NO + O/H Reaction (11)a/b
· Whereas, in fuel-rich systems (equivalence ratios more than about 1.2), the mechanism is through NH2:
HCN + OH  HNCO + H Reaction (12)
HNCO + H Reaction (12)
HNCO + H  NH2 + CO Reaction (13)
NH2 + CO Reaction (13)
NH2 + H  NH + H2 Reaction (14)
NH + H2 Reaction (14)
NH + H  N + H2 Reaction (10)
N + H2 Reaction (10)
N + O2/ OH  NO + O/H Reaction (11)a/b
NO + O/H Reaction (11)a/b

Table 1: Rate Coefficients (kj) for Prompt NO reactions in Arrhenius Form:
kj = ATâexp(-Eo /RT). Units are moles, cubic centimeters, seconds and Kelvin. (from Glaborg & Hadvig, 1991).
3. Characteristics of Prompt NO formation
Prompt NO has a weak temperature dependence, is very rapid, and occurs on a time scale comparable to energy release reactions (i.e., a reaction time of a couple of microseconds). However, it is very dependent on the [GLOSS]air-fuel stoichiometry[/GLOSS]. The amount of prompt NO is higher as the fuel/air ratio increases, being maximum in the fuel rich region: above an equivalence ratio of approximately 1.4, not enough oxygen radicals are however present to react with HCN and form NO. Therefore, prompt NO levels starts to decrease again. On the fuel-lean side of stoichiometry, few hydrocarbons fragments are available to react with atmospheric nitrogen to form HCN, which is the precursor of prompt NO.
4. When is prompt NO important?
Typical levels of prompt NO produced in stoichiometric premixed HC-air flames range from 50-90 ppmv, with the highest levels occurring for fuels with low H/C ratios, e.g., C2H2 and C6H6 (Bartok and Sarofim, 1991). During combustion in actual burners, the proportion of prompt NO in the NO emissions is estimated to be usually low, about 5%.
Table 2 lists estimations of the relative importance of prompt NO to nitrogen oxide emissions from practical combustion systems.
|
Device/fuel |
Estimated Percent of Prompt NO |
|
Utility boiler/natural gas |
17 |
|
Gas turbine/natural gas |
30 |
|
SI engine/gasoline |
10 |
|
CI engine/diesel fuel |
5 |
|
Utility boiler/coal (N=1%) |
<5 |
Table 2: The importance of Prompt NO in NOx emissions from practical combustion devices (from Bartok and Sarofim [4]).
At a stoichiometric equivalence ratio, in the turbulent flame of combustors, prompt NO is responsible for 30% of the total NOx emissions form gas turbines. However, for coals containing around 1% bound nitrogen, prompt NO has been estimated to be less than 5% of total NO and therefore, is generally considered as negligible when compared with fuel NO. When compared to thermal NO, the proportion of prompt NO is largest under cooler, [GLOSS]sub-stoichiometric[/GLOSS] conditions and short residence times see Figure 1.
With the new technology for reducing NOx emissions due to thermal and fuel NO mechanisms, the relative importance of prompt NO to nitrogen oxide emissions is increasing.
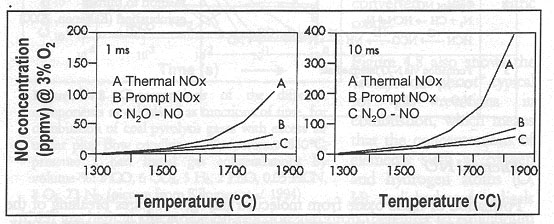
Figure 1: The significance of the different NO formation mechanism as related to temperature and residence time when burning methane under stirred reactor conditions (CSTR). Pressure 1 bar, stoichiometry ratio 1.15. Residence time 1 ms in the left hand diagram and 10 ms in the right hand diagram. (Kilpinen,1995; data from Glargorg, 1993) [5].
5. De Soete equation: reaction rate of prompt NO
De Soete [1a] proposes an estimated chemical reaction rate appropriate for the prompt NO formation mechanism [1b]. In terms of concentration, it is:
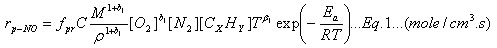
where M stands for the mixture molecular weight and ![]() is the mixture density.
is the mixture density.
The oxygen power b1 may vary between 0 and 1. For a temperature range of 1200 K to 2500 K, a value of 0.5 is appropriate. For the temperature power β1 , a value of 1 is advised.
For methane, CH4, which is the main component of natural gas, the constants Ci and Eai take the following values [1b]:
C=6.4*106 s-1
= 72.5*103 cal/mole
The prompt factor ![]() is calculated as follows:
is calculated as follows:

where the Nc is the number of carbon atoms in the hydrocarbon and  the local stoichiometry (air/fuel or air factor).
the local stoichiometry (air/fuel or air factor).
Acknowledgements
We would like to thank Dr. Kilpinen for her good advices and kind support.
Sources
[1a] De Soete, G.G.: Overall Reaction Rates of NO and N2 Formation from Fuel Nitrogen, 15th Symp. (Int’l.) on Combustion, p. 1093. The Combustion Institute, 1975.
[1b] Mancini, M., Weber, R., Bollettini, U.: Mathematical models development for design of HATC systems, 4th HTAG High Temperature Air Combustion and Gasification, Roma, 2001.
[2] Principles of Combustion Engineering for Boilers. Edited by C.J. Lawn, 1987.
[3]Glarborg, P., Hadvig, S.: Modelling and Chemical reactions: Development and Test of a KineticModel for Natural Gas Combustion, Report, Technical University of Denmark, 1991.
[4] Bartok W. , Sarofim A.F.: Fossil Fuel Combustion, 1991, USA.
[5]Zevenhoven R., Kilpinen P.: Control of pollutants in flue gases and fuel gases, Picaset Oy, Espoo, ISBN 951-22-5527-8, 2001 (e-book at www.hut.fi/~rzevenho/books.html)
