-
How is Basic Oxygen Furnace Gas (BOFG) produced?
Date posted:
-
-
Post Author
dev@edge.studio
1. Introduction
The typical fuel gases used in integrated iron and steelworks are listed in CF62 and introduced in more detail in CF100. This group of industrial fuel gases includes the byproduct of [GLOSS]Basic Oxygen Steelmaking[/GLOSS] ([GLOSS]BOS[/GLOSS]), referred to here as [GLOSS]Basic Oxygen Furnace Gas[/GLOSS] or [GLOSS]BOFG[/GLOSS], but also known as [GLOSS]Basic Oxygen Steelmaking Gas[/GLOSS].
BOFG is produced as a by-product of the process of decarburising liquid iron ([GLOSS]hot metal[/GLOSS]) in a [GLOSS]Basic Oxygen Furnace[/GLOSS], [GLOSS]BOF[/GLOSS]. Decarburised liquid iron is called liquid steel.
BOFG is a low to medium [GLOSS]calorific value[/GLOSS] fuel gas, which can be used as gaseous fuel in various applications inside and outside the steel plant.
The fuel properties of BOFG with its combustion and [GLOSS]flue Gas[/GLOSS] properties, and its applications are described in associated Combustion File numbers 245, and 247 respectively. The present CF concentrates on basic oxygen furnace gas production.
2. Production of Basic Oxygen Furnace Gas
The purpose of a basic oxygen furnace is to refine liquid iron (hot metal) into liquid steel. In table 1 typical compositions of iron and steel are given.
As by-products, [GLOSS]basic oxygen furnace slag[/GLOSS] and basic oxygen furnace gas are generated. The processes taking place in a basic oxygen furnace are described in numerous textbooks and articles. A good, comprehensive description is given in the article “The basic oxygen steelmaking BOS process”, written by John Stubbles [2]. In this section, this article is used to describe the production of Basic Oxygen Steelmaking Gas.
|
|
Iron |
Steel |
|
Fe |
94.60 % |
99.78 % |
|
C |
4.50 % |
0.03 % |
|
Mn |
0.40 % |
0.17 % |
|
Si |
0.40 % |
0.00 % |
|
P |
0.07 % |
0.01 % |
|
S |
0.04 % |
0.01 % |
Table 1 Typical compositions of iron and steel
Feedstocks
The primary raw materials for the Basic Oxygen Furnace process are 70-80% liquid hot metal from the blast furnace and the balance is steel scrap. These are charged into the Basic Oxygen Furnace vessel. Oxygen is blown into the BOF at supersonic velocities.
Energy Considerations
The oxygen oxidizes the carbon and silicon contained in the hot metal liberating great quantities of heat, which melts the scrap. There are lesser energy contributions from the oxidation of iron, manganese, and phosphorus. The post combustion of carbon monoxide as it exits the vessel also transmits heat back to the bath.
Products
The product of the BOF is molten steel with a specified chemical analysis at 1600°C-1650°C. From here it may undergo further refining in a secondary refining process or be sent directly to the continuous caster where it is solidified into semi finished shapes: blooms, billets, or slabs.
Process – See Figure 1
The refinery process begins when the BOF vessel is tilted about 45 degrees towards the charging aisle and scrap charge (about 25 to 30% w/w) is dumped from a charging box into the mouth of the cylindrical BOF. The hot metal is immediately poured directly onto the scrap from a transfer ladle.
Then the vessel is rotated back to the vertical position and lime/dolomite fluxes are dropped onto the charge from overhead bins while the lance is lowered to a few feet above the bottom of the vessel. The lance is water-cooled with a multi-hole copper tip. Through this lance, oxygen of greater than 99.5% purity is blown into the mix.
As blowing begins, an ear-piercing shriek is heard. This is soon muffled as silicon from the hot metal is oxidized forming silica, SiO2, which reacts with the basic fluxes to form a gassy molten slag that envelops the lance.
Blowing continues for a predetermined time based on the metallic charge chemistry and the melt specification. This is typically 15 to 20 minutes, and the lance is generally preprogrammed to move to different heights during the blowing period.
The lance is then raised so that the vessel can be turned down towards the charging aisle for sampling and temperature tests. Once the charge is ready for tapping and a preheated ladle is positioned in the ladle car under the furnace, the vessel is tilted towards the tapping aisle, and steel emerges from the taphole of the vessel.
The taphole is generally plugged with material that prevents slag entering the ladle as the vessel turns down. Steel burns through the plug immediately. To minimize slag carryover into the ladle at the end of tapping, various “slag stoppers” have been designed. Slag in the ladle results in phosphorus reversion, retarded desulphurisation, and possibly “dirty steel”. In Figure 2 a ladle with molten steel is shown.
After tapping steel into the ladle, and turning the vessel upside down and tapping the remaining slag into the “slag pot”, the vessel is returned to the upright position. In many shops residual slag is blown with nitrogen to coat the inside of the vessel. Near the end of a campaign, gunning with refractory materials in high wear areas may also be necessary. Once vessel maintenance is complete the vessel is ready to receive the next charge.
Production of BOFG
During blowing, Basic Oxygen Furnace Gas is generated by the partial oxidation of the carbon in the liquid iron to carbon monoxide. The gas flows via the top of the vessel into a shaft. As the blowing starts, a lot of air is entrained above the vessel and the gas is burned before entering the shaft. The flue gas creates an inert plug flow in the shaft and the piping system. This is done to remove the air in the shaft and piping system before a combustible gas enters this system.
After a while, a cover is lowered to prevent air intake and combustion. Gradually the CO content in the gas will rise. In the shaft, the untreated Basic Oxygen Steelmaking Gas is cooled from about 1400°C to about 900°C by generating steam.
The Basic Oxygen Steelmaking Gas is then cleaned and cooled in a series of scrubbers, electrostatic precipitators and/or bag filters. The carbon monoxide concentration in the Basic Oxygen Steelmaking Gas is not constant during this batch process. At the start and at the end of the process, the carbon monoxide concentration is very low. In these periods the gas is flared. In the period where the carbon monoxide concentration in the gas is sufficiently high (>30%), the gas is collected in a gasholder and can be used as gaseous fuel in furnaces and boilers.
Basic Oxygen Steelmaking Gas is produced as a by-product of the process of decarburising liquid iron in a [GLOSS]basic oxygen furnace[/GLOSS], BOF, vessel. Decarburised liquid iron is called liquid steel.
Basic Oxygen Steelmaking Gas is a medium [GLOSS]calorific value[/GLOSS] fuel gas, which can be used as gaseous fuel in various applications inside and outside the steel plant.
The fuel properties of BOS, its combustion and flue gas properties, and its applications are described in associated Combustion File numbers 245, 246 and 247 respectively.
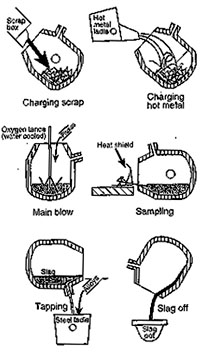

Figure 1 BOF vessel in its Figure 2 Ladle with molten steel
operating positions [3]
Sources
[1] American Iron and Steel Institute, www.steel.org.
[2] Stubbles, J., The basic oxygen steelmaking BOS process, www.steel.org.
[3] AISE Steel Foundation, 1998, Making, Shaping, and Treating of Steel, 11th Edition, Steelmaking And Refining Volume. Pittsburgh PA
[4] Zabetakis, M.G., Flammability characteristics of combustible gases and vapors, Bulletin 627, Bureau of Mines, 1965.
[5] N.V. Nederlandse Gasunie, Physical properties of natural gases, Groningen, Netherlands, 1980.
[6] Informatie Chemische Stoffen, werkinstructiekaart oxygas, Corus, 2002.
