-
What are trace metal emissions?
Date posted:
-
-
-
Post Author
Patrick LaveryCombustion Industry News Editor
-
1. General
In combustion systems, important [GLOSS]trace metal[/GLOSS]s from an emissions point of view are: Antimony (Sb), Arsenic (As), Barium (Ba), Beryllium (Be), Boron (B), Cadmium (Cd), Chromium (Cr), Cobalt (Co), Copper (Cu), Iron (Fe), Lead (Pb), Manganese (Mn), Mercury (Hg), Molybdenum (Mo), Nickel (Ni), Potassium (K), Selenium (Se), Sodium (Na), Thallium (Tl), Tin (Sn), Uranium (U), Vanadium (V) and Zinc (Zn), although technically any metal present in trace amounts can be accounted as a trace metal.
In some sources, the concentrations of the above listed trace metals may exceed 0.1% wt, but they are still considered as trace metals.
2. Nature of Emissions
Trace metal emissions from [GLOSS]combustion[/GLOSS] processes may be gaseous (for the most volatile metals, e.g. Hg), or be present in [GLOSS]ash[/GLOSS], particularly in fine ash. Those present in ash can be partially removed by [GLOSS]particulate control devices[/GLOSS].
Generally, the trace metals can be separated into three classes according to their volatility:
Class I: Those that do not volatilise during combustion or gasification, and distribute more or less evenly over [GLOSS]bottoms ash[/GLOSS] and [GLOSS]fly ash[/GLOSS], e.g. Mn.
Class II: Those that vaporise but are found mainly in fly ash, after condensation on [GLOSS]particulate matter[/GLOSS] and nucleation, as a result of decreasing temperatures of the flue gas in the flue gas duct, e.g. As, Cd, Pb, Sn, Zn, Cr.. A small but significant proportion of trace metals are present in fine particulate matter in the sub-micron (<1.0mm) range where [GLOSS]particulate control devices[/GLOSS] are less effective. For example, approximately 35% of the total arsenic emissions can be present in the sub-micron range of fine ash, and 20% for chromium.
Class III: Those that vaporise and only partially condense before direct gaseous release into the atmosphere, e.g. Hg, and to some extent Se and B.
Figure 1 depicts the classes of trace metals.
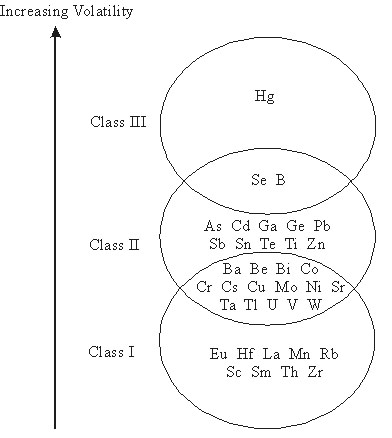
Figure 1. Classes of trace metals according to volatility (adapted from Couch, 1995).
Those trace metals released into the environment can be deposited onto land or aquatic surfaces, and be consumed by livestock, fish or even plant matter and eventually find their way into the human food chain.
3. Adverse Effects
Health Effects
The United States [GLOSS]EPA[/GLOSS] lists eleven trace metals as hazardous air pollutants ([GLOSS]HAPs[/GLOSS]): Hg, Se, As, Cd, Sb, Ni, Cr, Co, Pb, Be and Mn. The European Community includes all of these metals in their list of 13 metals of highest concern, adding vanadium and thallium to the list.
The physiological effects of trace metals on humans are principally effects on the central nervous system (Hg, Pb, Se, As), the kidneys and/or liver (Hg, Pb, Se, Cd, Cu), and the skin, bones and teeth (Ni, Sb, Cd, Se, Cu, Cr).
Environmental Effects
Trace metals occur naturally in the ecosystem in varying concentrations, partly as the result of pollution. Elevated levels of trace metals can be toxic to flora and fauna, affecting growth, fertility and in the most extreme cases causing death. However, as with humans, some flora and fauna require trace amounts of some metals to grow and survive (these are micronutrients).
Effects on Process Equipment
Trace metals may affect various types of process equipment in a number of different ways. Corrosion is a common problem caused by the anions to trace metals (e.g. anions to Na, K, V, Zn, Pb). Arsenic and potassium can act as a catalyst deactivator. Fuel cell electrodes and electrolytes can be deactivated by small amounts of trace metals.
4. Sources of Trace Metals
The primary source of trace metal emissions in combustion systems is the fuel. If other feedstocks contain trace metals, these may contribute to the emissions. Table 1 lists typical trace metal concentrations of various fuels. As additional information, it should be noted that leaded gasoline/petrol can contain around 5mg/Litre Pb. For coal, emissions of trace metals are closely tied to trace metal associations.
|
|
[GLOSS]Coal[/GLOSS] |
[GLOSS]Peat[/GLOSS] |
[GLOSS]Heavy Fuel Oil[/GLOSS] |
[GLOSS]PET coke[/GLOSS] |
[GLOSS]MSW[/GLOSS] |
[GLOSS]WDF[/GLOSS] |
[GLOSS]Wood[/GLOSS] |
[GLOSS]Waste Wood[/GLOSS] |
[GLOSS]Waste Paper[/GLOSS] |
[GLOSS]Scrap Tyres[/GLOSS] |
[GLOSS]Sewage Sludge[/GLOSS] |
Hg |
0.02 – 3 |
~ 0.07 |
< 0.01 |
|
<15 |
1 – 10 |
0.01 – 0.2 |
|
~ 0.08 |
|
0.5 – 10 |
|
As |
0.5 – 10 |
1 –3 |
1 – 2 |
|
0.5 – 500 |
~ 3 |
~ 0.2 |
|
|
|
0.1 – 100 |
|
Be |
0.1 – 10 |
~ 0.1 |
~ 0.01 |
|
1 – 40 |
~ 1 |
|
|
~ 0.8 |
|
|
|
Cd |
0.05 – 10 |
|
|
0.1 – 0.3 |
< 100 |
1 – 10 |
|
~ 0.5 |
~ 0.7 |
5 – 10 |
1 – 10 |
|
Co |
0.5 – 20 |
1 – 2 |
~ 0.5 |
|
< 20 |
|
~ 0.1 |
|
|
|
~ 5 |
Cr |
0.5 – 60 |
0.5 – 2 |
~ 0.5 |
5 – 104 |
< 1500 |
50 – 250 |
~ 1 |
1 – 4 |
~ 6 |
~ 100 |
~ 100 |
|
Cu |
5 –60 |
~ 10 |
< 0.1 |
|
< 2500 |
< 1000 |
0.5 – 3 |
~ 15 |
~ 18 |
|
200 – 700 |
|
Mn |
5 – 300 |
30 – 100 |
0.5 – 1 |
|
< 1000 |
~ 250 |
10 – 1000 |
|
~ 27 |
|
~ 200 |
|
Ni |
0.5 – 100 |
5 – 10 |
20 – 50 |
200 – 300 |
< 5000 |
10 – 100 |
~ 0.5 |
< 20 |
~ 7 |
~ 75 |
~ 50 |
|
Pb |
1 – 300 |
1 – 5 |
1 – 5 |
6 – 100 |
< 2500 |
100 – 500 |
1 – 20 |
< 50 |
~ 8 |
60 – 760 |
100 – 300 |
|
Sb |
< 1 |
|
|
|
< 80 |
< 5 |
|
|
~ 5 |
|
100 – 500 |
|
Se |
0.2 – 3 |
~ 1 |
~0.1 |
|
< 10 |
3 – 6 |
~ 0.2 |
|
~ 0.08 |
|
|
|
Sn |
< 10 |
|
|
|
3 – 100 |
~ 500 |
|
|
~ 8 |
|
|
|
Tl |
~ 1 |
|
|
0.04 – 3 |
|
|
|
|
|
~ 0.25 |
|
|
V |
1 – 100 |
5 – 50 |
100 – 200 |
400 – 900 |
|
|
~ 2 |
|
|
|
|
|
Zn |
1 – 1000 |
~ 20 |
~ 10 |
|
~ 2% |
300 – 800 |
5 – 150 |
< 30 |
~ 150 |
1 – 2% |
~ 1000 |
* Units: mg/kg, ie ppmw, moisture free.
Table 1: Typical concentrations of trace metals in various fuels (Zevenhoven and Kilpinen, 2001)
The route that trace metals take from the source to being emitted is not in the scope of this combustion file. Further combustion files (CF59, CF188, CF189) deal with further issues relating to mercury.
Sources
[1] Zevenhoven, R. and Kilpinen, P., Control of Pollutants in Flue Gases and Fuel Gases, Helsinki, 2001
[2] Couch, G.R., Power from Coal – where to remove impurities, London, 1995
[3] Swaine, D.J., Why trace elements are important, Fuel Processing and Technology, 2000
[4] Senior, C.L. et al., Emissions of mercury, trace elements, and fine particles from stationary combustion sources, Fuel Processing Technology, 2000.
[5] Benson, S.A. et al., Trace Metal Transformation in Gasification. Presented at the Advanced Coal-Fired Power Systems ’96 Review Meeting, Morgantown, WV, July 16-18, 1996.
