-
What are the properties of black liquor?
Date posted:
-
-
Post Author
dev@edge.studio
1. Introduction
[GLOSS]Black liquor[/GLOSS] is a fuel of the [GLOSS]chemical pulping[/GLOSS] industry and has many special features different from other fuels. This CF lists and explains these special features that are either properties of the black liquor or a property important for the handling and burning of it and a property that has to be taken into account in the recovery of the chemicals inside the recovery boiler. Some of the properties (e.g. chemical composition and physical properties) were included in CF199.
The properties for black liquor can be named as for other fuels but the special feature of recovering the inorganic pulping chemicals results in that some properties can not be used in the same way as for other fuels, e.g. “black liquor ash” is not in practice a valid term but it should be called either flue gas dust, or [GLOSS]inorganic smelt[/GLOSS], depending on if the inorganic residue is in the flue gas stream or in the smelt bed on the bottom of the furnace. The main properties of the smelt have been included in this CF.
2. Black liquor properties
Composition
Black liquor comprises about half wood organics and half inorganic pulping chemicals. It contains water and dry solids. The main components of the [GLOSS]dry solids[/GLOSS] are organic substances dissolved from wood during [GLOSS]cooking[/GLOSS] ([GLOSS]lignin[/GLOSS]s, [GLOSS]hemicellulose[/GLOSS], [GLOSS]cellulose[/GLOSS]) and inorganic substances (sodium and sulfur compounds). Minor inorganic components are potassium and chlorine compounds. Elemental composition and organic chemical species are given in CF199.
Physical properties
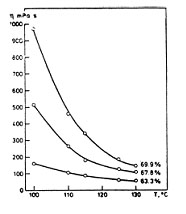
Viscosity – Black liquor is a non-Newtonian fluid and its apparent viscosity is a function of concentration and temperature and specific for each liquor. It depends on the wood species, cooking method and several other subprocesses of the pulp mill and thereby it varies considerably from mill to mill. Available formulas for calculating viscosity primarily use statistical data. The viscosity increases with increasing dry solids content of the liquor and decreases with increasing temperature (Figure 1, left part). The practical limit for handling the black liquor is the pumping limit of 300-500cp which results in a limit of dry solids of 70-75% and storing temperature of 115°C if the storage is done at atmospheric pressures. Modern mills have started to use pressurized storage for the final evaporated liquor and can thereby increase the dry solids content. Viscosity can in some cases be lowered by e.g. heat treatment processes used in the evaporation plant. The heat treatment at ~180°C mainly breaks the long organic molecules into shorter chains, thereby decreasing irreversibly the viscosity of the black liquor.
Viscosity influences the specification of equipment and the use of energy for storing and transporting the black liquor. It also influences the droplet formation process inside the recovery boiler in the immediate vicinity of the [GLOSS]liquor gun[/GLOSS]s.
|
|
|
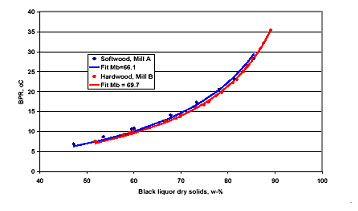
Figure 1. Viscosity of one liquor at different dry solids and temperature (left) and boiling point rise (right) for two mill liquors.
Boiling point rise (BPR) – A liquid mixture that contains dissolved organic substances, inorganic substances, or both, will boil at a higher temperature than water at the same pressure. This temperature difference is called the boiling point rise (BPR). BPR is specific for each liquor and depends on the amount and composition of the dissolved substances. BPR increases with increasing dry solids (Figure 1, right part) and influences the steam properties needed in the evaporation plant.
Density – Density increases with dry solids and can be calculated using formulas based on statistical data. A black liquor of 20% and 70%DS has viscosities (at 90°C) of 1.05 t/m3 and 1.43 t/m3, respectively. There is a minor influence of temperature. Density influences mainly design and dimensioning of the equipment.
Surface tension – The practical implication of the surface tension is the foaming tendency of the black liquor at lower concentration in the evaporation plant. Foaming decreases the performance of the evaporation plant. [GLOSS]Crude tall oil[/GLOSS] and [GLOSS]black liquor soap[/GLOSS] will decrease the surface tension and it is good to separate them from the black liquor before the evaporation plant.
Specific heat – Specific heat is dependent of the heat capacities of the organic and inorganic components of the black liquor. It decreases with increasing dry solids. It is mainly needed to calculate energy need in the evaporation plant and in storage.
Solubility of inorganic substances in black liquor – The inorganic substances in the black liquor are mostly bound to the dissolved organic compounds. A minor portion of the inorganic substances such as NaOH, Na2S, Na2CO3, Na2SO4, etc. dissolve as salts in the weak black liquor but Na2CO3 and Na2SO4 (or their double salt 2Na2SO4*Na2CO3, burkeite) start to precipitate at about 45-60% DS during concentration. Normally the pH of weak liquor is about 12 but if process disturbances decrease the residual alkaline material (NaOH and Na2S) the pH decreases to below 11 and the lignin starts to precipitate.
The formation of burkeite precipitates can start rapidly and foul the heat transfer surfaces disturbing the evaporation process. The heating surfaces are, though, easily washable.
3. Smelt and its properties
Smelt is the product of the inorganic reactions in the recovery boiler and is the main substance to be recovered from the recovery boiler. The smelt originates in the inorganic chemicals from the black liquor and would for other fuels be called “ash”. The amount of smelt is about 0.4 kg /kg dry solids.
The actual properties of smelt are extremely difficult to measure due to the high temperatures. It is possible to analyze the chemical composition but the sampling technique is decisive in gathering a representative sample.
Composition
The main components are Na2CO3 (~70%) and Na2S (~25%), minor amounts (<1%) of Na2SO4 and Na2S2O3 are found, other components (~5%) being mainly the respective potassium salts and chlorine salts and some insoluble constituents.
Reduction – The main process property is reduction of the smelt. It is the molar ratio of Na2S to (Na2S and Na2SO4) and tells how much of the sulfur exits the boiler as sodium sulfide that is usable in the cooking process. The Na2SO4 is a dead load in the cooking process.
Properties
Viscosity – Smelt viscosity is dependent of melting range and temperature. Recent successful first smelt viscosity measurements gives numbers to e.g. estimate how easily the smelt can flow out from the recovery boiler.
Melting range – Melting of inorganic mixtures does not happen at one temperature like the melting of a pure chemical. Melting takes place over a temperature range which is dependent on the concentration of the individual components. Pure Na2CO3 melts at around 853°C and Na2S at around 1150C but a mixture of 90 mole-% Na2CO3 and 10 mole-% Na2S start melting at 760°C and is fully molten first at about 830°C.
Smelt composition and melting range heavily influences the viscosity.
Sources
T. N. Adams (Ed.), Kraft Recovery Boilers, Tappi Press, 1997.
E. Vakkilainen, Kraft Recovery Boilers – Principles and Practice, Lappenranta University, 2001.