-
What are the main NOx formation processes in combustion plant?
Date posted:
-
-
Post Author
dev@edge.studio
1. Background
In order to present a clear background to the continuously developing range of
Nitrogen Oxides ([GLOSS]NOx[/GLOSS]) control techniques and equipment, it is essential to understand NOx formation chemistry.
There are a number of nitrogen oxides, but only three of these are of interest for combustion processes:
- Nitrogen monoxide, or nitric oxide, NO
- Nitrogen dioxide, NO2
- Di-nitrogen oxide, or nitrous oxide, or “laughing gas”, N2O
The first two, NO and NO2 are collectively referred to as NOx and they are essential contributors to the acid rain or smog problems.
The NOx content in the combustion gases from conventional power plant boilers and many industrial heating process contains some 90% NO with the remainder
NO2.
The third oxide, N2O, is found in flue gases from, among others, Fluidised bed combustors, and from engine exhaust gases after the catalytic converting system.
N2O is not an acidic oxide, and is normally not included in the abbreviation NOx. However,
N2O is a gas, which contributes to the destruction of the stratospheric ozone.
This Combustion File gives a brief overview on this subject and gives a platform for more detailed Combustion Files both on NOx formation and reduction chemistry.
There are basically three recognized mechanism on NOx formation – Thermal, Fuel and Prompt. These are outlined below.
2. Thermal NOx formation
Thermal NOx is produced by the reaction of atmospheric oxygen and nitrogen at elevated temperatures, and is reputed to contribute about 20% of the total NOx emission in pulverised coal firing, but is the dominant mechanism when the fuel contains little or no inherent nitrogen (i.e. gas firing). Where high air preheat temperatures are employed, for example in cement kilns, thermal NOx can also contribute considerably to the overall NOx emission.
The reactions are described by the Zeldovich mechanism as follows:
N2 + O = NO + N
(1)
N + O2 = NO + O
(2)
N + OH = NO + H
(3)
The first step is rate limiting, and due to its high activation energy (314 KJ/mol) requires high temperatures to proceed. Reaction (3) is only significant under reducing conditions.
By kinetic analysis it is possible to derive an overall expression for the rate of thermal NOx formation (Bowman, 1975), viz:
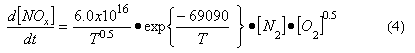
Where
T = absolute temperature (K)
[N2], [O2] = concentration of nitrogen, oxygen (mol/cm3)
d[NOx]/dt = rate of NOx formation (mol/cm3/s)
In practise the control/minimisation of thermal NOx is accomplished primarily by measures, which reduce temperature, but dilution of the available oxygen is also beneficial.
3. Fuel NOx formation
Fuel NOx arises from the reaction of the organically bound nitrogen in the fuel with oxygen. The process is complex (reaction schemes typically consider of the order of 50 intermediate species and several hundred separate reversible reactions, and there is still considerable uncertainty as to the true value of the various rate constants, etc.), but can be simply expressed as follows:
- Volatile fuel nitrogen is evolved mainly as HCN (and NH3) during the processes.
- The HCN reacts with various free radical species (O, OH) to form intermediates such as CN, NCO, HNCO and ultimately with reaction with H to produce NH,
NH2.
Fuel NOx can be most effectively minimised by burning the fuel by staged combustion, which implies delayed mixing between the fuel gas and air.
4. Prompt NOx formation
Prompt NOx is formed by the reaction of hydrocarbon radicals with atmospheric nitrogen to produce HCN and hence NOx via a complex series of gas phase reactions. The contribution of the prompt NOx to the total emission in pulverised coal combustion is small (about 5%). Measures, which are effective in minimising thermal and fuel NOx, are also effective in minimising prompt NOx.
Sources
[1] Bowman CT, Kinetics of Pollutant Formation and Destruction on Combustion, Prog Energy Combust Sci 1 33-45, (1975).
[2] Beltagui SA, Kenbar AMA & McCallum NRL, NOx Generation and Control in Confined Swirling Flames – Review and Parametric Study, HTFS Paper No RS 827, (1989).
