-
What are the combustion and flue gas properties of Coke Oven Gas?
Date posted:
-
-
Post Author
dev@edge.studio
1. Introduction
The typical fuel gases used in integrated iron and steelworks are listed in CF62 and introduced in more detail in CF100. This group of industrial fuel gases includes [GLOSS]Coke Oven Gas[/GLOSS] or [GLOSS]COG[/GLOSS].
COG is produced as a by-product from [GLOSS]coke[/GLOSS] production. COG is valuable since it can be used as a relatively rich gaseous fuel in various applications inside or outside the steel plant. One of the biggest consumers is the coke plant itself, where up to 50% is used as the coke plant fuel.
The production of COG, its fuel gas properties, and its the applications are described in associated Combustion File numbers 217, 239 and 241 respectively.
The present combustion file provides information on the combustion and flue gas properties of Coke Oven Gas.
2. Combustion and flue gas properties of Coke Oven Gas
Combustion properties that are important when Coke Oven Gas is fired in industrial furnaces and boilers are:
q The [GLOSS]stoichiometric air requirement[/GLOSS];
q The [GLOSS]adiabatic flame temperature[/GLOSS];
q The [GLOSS]flammability limits[/GLOSS],
q The [GLOSS]auto-ignition temperature[/GLOSS];
q The [GLOSS]flue gas[/GLOSS] composition.
Stoichiometric Air Requirement
For burning Coke Oven Gas under stoichiometric conditions, the air requirement varies between 4.2 and 4.9 mo3/mo3 of COG.
This is a relatively small air requirement compared to [GLOSS]Natural Gas[/GLOSS] – NG (typically 8.5 mo3/mo3 to 10.0 mo3/mo3 Natural Gas)
This is due to the fact that the main combustible component of COG is hydrogen – see CF239.
Adiabatic Flame Temperature
The high hydrogen concentration in COG results in a relatively high adiabatic flame temperature of 2100°C to 2130°C.
This is typically 100°C higher than the adiabatic flame temperature of a natural gas flame at stoichiometric conditions. Therefore, special attention should be paid to thermal NOx formation when Coke Oven Gas is applied.
Flammability Limits
A Coke Oven Gas – air mixture at ambient temperature and pressure is explosive if the concentration of Coke Oven Gas in the mixture is 4.5 – 35.5 vol.% [7].
The flammability limits of Coke Oven Gas are significantly wider than the flammability limits of natural gas (6.5 – 16.8 vol.%), due to the high amount of hydrogen.
Auto-Ignition Temperature
The auto-ignition temperature of a gas mixture is determined by the lowest auto-ignition temperature of any of the species in the gas mixture. In general, this is the hydrocarbon component with the largest molecule or hydrogen.
In a Coke Oven Gas – air mixture, the auto-ignition temperature of hydrogen determines the auto-ignition temperature. This is 400°C, which is relatively low compared to the auto-ignition temperature of natural gas: 617°C [3].
Flue Gas Composition
In Table 1 the flue gas compositions are presented for Coke Oven Gas flames fired stoichiometrically with complete combustion in a number of steel plants. The plants in this Table are the same plants as indicated in Table 1 of CF239.
|
Component |
|
Plant 1 |
Plant 2 |
Plant 3 |
Plant 4 |
Average |
SD |
|
Carbon Dioxide |
%v/v |
7.5 |
7.9 |
7.3 |
7.3 |
7.5 |
0.3 |
|
Nitrogen |
%v/v |
68.8 |
8.7 |
68.2 |
68.3 |
68.5 |
0.3 |
|
Water Vapour |
%v/v |
22.9 |
2.6 |
23.7 |
23.6 |
23.2 |
0.5 |
|
Argon |
%v/v |
0.8 |
0.8 |
0.8 |
0 .8 |
0.8 |
0.0 |
Table 1 Flue gas properties of stoichiometrically fired Coke Oven Gas flames, assuming complete combustion
An overview of the gas, combustion and flue gas properties of a typical Coke Oven Gas is shown in Table 2 below.
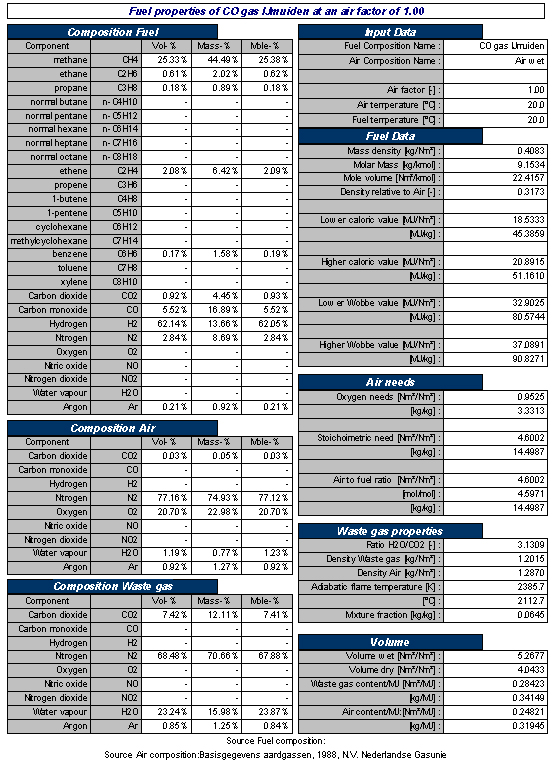
Table 2 Typical example of properties of Coke Oven Gas and combustion related properties at stoichiometric combustion
Sources
[1] American Iron and Steel Institute, www.steel.org.
[2]Wilson, H. Fuels and Combustion, Reheating Technical Course.
[3] N.V. Nederlandse Gasunie, Physical properties of natural gases, Groningen, Netherlands, 1980.
[4] Militzer, M.R., UEC Coal & Coke Laboratory, Coke Production for Blast Furnace Ironmaking.
[5] Valia, H.S. Coke production for blast furnace ironmaking, Ispat Inland Inc, American Iron and Steel Institute.
[6] Platts, M. The coke oven by-product plant, ThyssenKrupp EnCoke, American Iron and Steel Institute.
[7] Zabetakis, M.G., Flammability characteristics of combustible gases and vapors, Bulletin 627, Bureau of Mines, 1965.
[8] Informatie Chemische Stoffen, werkinstructiekaart oxygas, Corus, 2002.
