-
How are DIOXINS formed in waste incineration?
Date posted:
-
-
Post Author
dev@edge.studio
1. General
A number surveys on the inventories of dioxins emissions into atmosphere indicate substantial contribution of industrial processes such as combustion, metals smelting and refining and chemical manufacturing. Among these, waste incineration has been the dominant source. Formation mechanisms of dioxins in the process of waste incineration have been thus widely studied. These studies have provided a lot of understanding on the extreme complexity of the mechanisms behind the formation of dioxins.
2. Dioxins formation in a waste incinerator
Various mechanisms have been proposed for the dioxins emissions from incinerators. Figure 1 shows four possible formation paths identified:
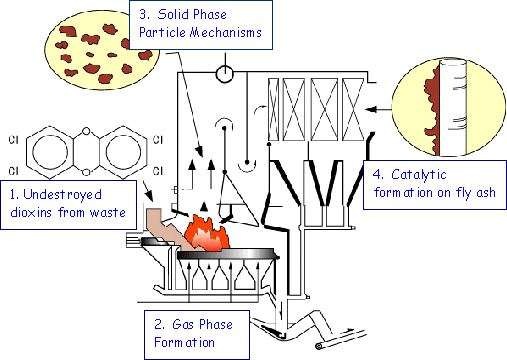
The second mechanism is more plausible, so-called gas-phase precursor formation. It involves homogeneous gas-phase reaction where chloro-phenols and PCB’s are the well-known precursors. A set of two di-chloro-phenols might combine to directly form TCDD. PCB can directly form TCDF with addition of one atom of oxygen. Preferred temperature is 500-700°C.
The third mechanism relates to the formation of dioxins from non-chlorinated organic compounds in waste particles that have similar structures to dioxins. Lignin, contained in paper or wood, has benzene rings and can form dioxins if split and chlorinated. Preferred temperature is 500-700°C.
The fourth mechanism relates to the heterogeneous catalytic formation, as opposed to the gas phase homogeneous formation. This formation mechanism is generally called ‘De-novo’ synthesis and is the most important. ‘De-novo’ is a Latin word meaning somewhat ‘new from scratch’. Catalytic compounds containing heavy metals on fly-ashes, with copper most active, play a key role. Through the catalytic reaction dioxins are believed to be formed from various carbon-containing organics in flue gas, with unburned carbons in fly-ashes most likely the major material. Favored temperature range is roughly between 150-400°C for this ‘de-novo’ catalytic formation, with ca. 300°C most active. This catalytic formation is most observed in flue gas cleaning devices such as ESPs (electro-static precipitator) where fly-ashes as well as flue gas reside at relatively low temperature with long residence time. Before dioxins problem was identified the most common temperature of ESPs used to be around 300°C, which was an unexpected coincidence and tragedy.
Sources:
Chlorinated Dioxin and Furan Formation, Control and Monitoring –
B. Gullett(USEPA) and R. Seeker(GEEERC), ICCR Meeting (Nov. 1997)
