-
IFRF-supervised student to investigate the ‘Allam-Fetvedt Cycle through Centre for Doctoral Training
Date posted:
-
-
-
Post Author
Philip SharmanIFRF Director
-
-
![]()
As many IFRF members will know, a particular natural gas fired power generation cycle that uses supercritical carbon dioxide (sCO2) as the primary process fluid has attracted considerable interest in recent years as offering a potential route to net-zero greenhouse gas emissions. The power cycle – invented by British engineer Rodney Allam and called the ‘Allam-Fetvedt Cycle’ – also offers high-efficiency and low-cost electricity generation and has been successfully demonstrated at 50MWth scale at La Porte, Texas, USA. The cycle is currently being commercialised by NET Power LLC, with 8 Rivers Capital leading the development of full-scale commercial projects.
What you may not know is that IFRF is acting as industrial sponsor/supervisor for an Engineering Doctorate student based at the University of Sheffield who is enrolled in the EPSRC Centre for Doctoral Training (CDT) for Resilient Decarbonised Fuel Energy Systems (see a previous IFRF blogpost from February 2019). The student – James Harman-Thomas – whom we introduced to you in a Monday Night Mail piece in March this year, will be studying (experimentally and theoretically) the chemical kinetic mechanism of methane combustion under supercritical CO2 conditions.
As promised in March, James has prepared an introduction to his proposed research – see below. As members of IFRF, if you or your organisations would like to link with James to help advise or steer them in his research, please let Philip Sharman know and he will put you in touch.
Theoretical and Experimental Investigation into the Chemical Kinetic Model for Supercritical CO2 Production
James Harman-Thomas
University of Sheffield
The Allam Cycle
The Allam cycle is a thermodynamic cycle, invented by Rodney Allam MBE, which has the potential to intrinsically capture 100% of its emissions and is now being developed and implemented by NetPower (Allam et al., 2017). The Allam cycle combusts natural gas, or synthesis gas (‘syngas’) produced from coal gasification at 300 bar and temperatures exceeding 1000 K. The novelty of the Allam cycle is that the fuel gas and oxygen are diluted in approximately 94% supercritical carbon dioxide (sCO2). As well as diluting the combustion reaction and moderating the combustion temperature and flame speed, sCO2 also acts as the working fluid which powers the turbine to generate electricity. Utilising sCO2 as the working fluid is advantageous as it is more energy-dense than the traditional steam and gas working fluids used to power turbines which leads to an improved efficiency of electricity production. The carbon dioxide (CO2) is separated from the water produced during combustion via condensation of the water to give a high-purity stream of high-pressure CO2. The majority of the CO2 is recycled into the combustion chamber and a small amount corresponding to that produced during combustion is removed. The CO2 removed can be stored or utilised in technologies such as enhanced oil recovery. Furthermore, the cost of electricity to the end user is expected to be cost-competitive with current fossil fuel power plants without carbon capture and storage. A schematic of the Allam cycle is displayed in figure 1.
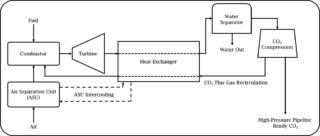
Figure 1. Simplified schematic of the Allam cycle.
NetPower have already built a 50 MW natural gas pilot plant in La Porte, Texas and are aiming to begin operation of a 300 MW demonstration plant in 2022.
Scope of Experimental Research
Despite a pilot plant utilising the Allam cycle already being commissioned, the chemical kinetic mechanism for the combustion of natural gas and syngas is poorly understood and little experimental data exists to validate existing current chemical kinetic mechanisms. The overall goal of this EngD project is to undertake experimental and theoretical work to produce a chemical kinetic model which more accurately replicates the experimental data recorded in supercritical CO2. The project will initially focus on methane combustion as it is the main constituent of natural gas and Allam cycle plants which burn natural gas are currently more developed than coal fired Allam cycle plants. However, the research aims for this project and beyond are to study other components of natural gas such as ethane and propane, as well as syngas and hydrogen in preparation for the development of plants burning syngas from coal gasification.
Theoretical Objectives
The first theoretical objective of my research is to write a critical review of current chemical mechanisms and existing experimental work at conditions relevant to the Allam cycle. The existing experimental work will be compared to chemical kinetic mechanisms which were produced for and validated using data recorded around atmospheric pressure and small CO2 concentrations. Comparison of the existing mechanisms will be performed through sensitivity analysis and reaction path analysis. All theoretical modelling work will be performed using ANSYS ChemKin Pro. This will provide valuable information in determining which reactions have the largest influence on the combustion properties such as ignition delay time and laminar flame speed at different pressures, CO2 dilutions and equivalence ratios for different fuel types. This information will be used to determine which reactions are important to study experimentally in order to accurately determine the pressure and temperature dependence of rate coefficients at conditions relevant to the Allam Cycle.
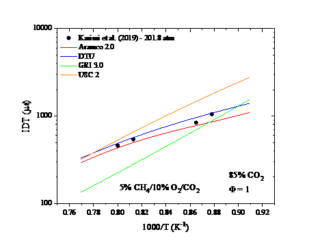
Figure 2. The IDT as a function temperature. Experimental data taken from Karimi et al. (2019).
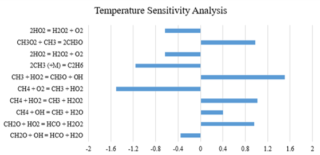
Figure 3. Temperature sensitivity data of methane combustion mole fraction CH4 (5):O2 (10):CO2 (85) at 201.8 atm and 1156 K for Aramco 2.0.
Figure 2 shows an example of such data. The performance of four different mechanisms Aramco 2.0, DTU, GRI 3.0 and USC Ⅱ for a set of ignition delay time measurements can be seen in figure 2 and a temperature sensitivity analysis of one of the two better performing mechanism at one temperature point for the best performing mechanism, Aramco 2.0 can be seen in figure 3. A positive sensitivity coefficient indicates that increasing the rate coefficient would reduce the ignition delay time and a negative sensitivity coefficient would increase the ignition delay time.
Experimental Objectives
The primary experimental objectives of this project are to make measurements of ignition delay time at different conditions, which can be used for the validation of the chemical mechanisms. The secondary objective is to determine the rate coefficient of reactions identified as important through the sensitivity analysis and fitting rate coefficients of relevant rate coefficients to species time histories. The theoretical determination of rate constants for these reactions already diluted in CO2 is already underway, however, to develop a comprehensive mechanism, all of the important reactions involved in combustion must be determined. From this, the reactions that are less important to ignition delay time can be estimated using experimental data or approximations based of similar reactions.
The experiments will be performed following the construction of a shock tube at the forthcoming Translational Energy Research Centre (TERC) facility at the University of Sheffield. The shock tube will be able to achieve pressures of up to 200 bar and will employ a series of pressure and species diagnostics in order to simultaneously make ignition delay time measurements and record species time histories. When studying combustion with significant dilutions of polyatomic bath gases such as CO2 the results can suffer due to the effects of bifurcation. Bifurcation occurs when a boundary layer, a thin layer of gas that forms on the shock tube walls interacts with the reflected shock and perturbs the test gas from the ideal constant pressure and temperature assumption. One experimental objective will be to lessen the bifurcation effect, which reduces the test times achievable in shock tube experiments, as well as reduce the accuracy of experimental measurements.
Summary
The overall objectives of my EngD project are to review the existing data and combustion mechanisms before constructing a shock tube experimental technique to collect more high-pressure data at conditions relevant to sCO2 combustion. The extensive critical review and new experimental data will be used to develop a chemical kinetic mechanism which better replicates experimental data at high-pressures and high dilutions of CO2.
I would like to thank Mr Philip Sharman and the IFRF for their supervision of my project and I look forward to becoming involved with the IFRF community and sharing my results in future editions of the Monday Night Mail and as papers in the Industrial Combustion journal.
References
ALLAM, R., MARTIN, S., FORREST, B., FETVEDT, J., LU, X. J., FREED, D., BROWN, G. W., SASAKI, T., ITOH, M. & MANNING, J. 2017. Demonstration of the Allam Cycle: An update on the development status of a high efficiency supercritical carbon dioxide power process employing full carbon capture. 13th International Conference on Greenhouse Gas Control Technologies, Ghgt-13, 114, 5948-5966.
ALLAM, R. J., PALMER, M. R., BROWN, G. W., FETVEDT, J., FREED, D., NOMOTO, H., ITOH, M., OKITA, N. & JONES, C. 2013. High efficiency and low cost of electricity generation from fossil fuels while eliminating atmospheric emissions, including carbon dioxide. Ghgt-11, 37, 1135-1149.
HARGIS, J. W. & PETERSEN, E. L. 2015. Methane Ignition in a Shock Tube with High Levels of CO2 Dilution: Consideration of the Reflected-Shock Bifurcation. Energy & Fuels, 29, 7712-7726.
HASHEMI, H., CHRISTENSEN, J. M. & GLARBORG, P. 2019. High-pressure pyrolysis and oxidation of DME and DME/CH4. Combustion and Flame, 205, 80-92.
KARIMI, M., OCHS, B., LIU, Z. F., RANJAN, D. & SUN, W. T. 2019. Measurement of methane autoignition delays in carbon dioxide and argon diluents at high pressure conditions. Combustion and Flame, 204, 304-319.
METCALFE, W. K., BURKE, S. M., AHMED, S. S. & CURRAN, H. J. 2013. A Hierarchical and Comparative Kinetic Modeling Study of C1 − C2 Hydrocarbon and Oxygenated Fuels. International Journal of Chemical Kinetics, 45, 638-675.
RODRÍGUEZ HERVÁS, G. & PETRAKOPOULOU, F. 2019. Exergoeconomic Analysis of the Allam Cycle. Energy & Fuels, 33, 7561-7568.
SMITH, G. P., GOLDEN, D. M., FRENKLACH, M., MORIARTY, N. W., EITENEER, B., GOLDENBERG, M., BOWMAN, C. T., HANSON, R. K., SONG, S., GARDINER JR., W. C., LISSIANSKI, V. V. & QIN, Z. n.d. GRI Mechanism 3.0 [Online]. combustion.berkeley.edu: University of California, Berkeley. Available: http://combustion.berkeley.edu/gri-mech/version30/text30.html[Accessed 16th December 2019].
WAIT, E. E., MASUNOV, A. E. & VASU, S. S. 2019. Quantum chemical and master equation study of OH + CH2O → H2O + CHO reaction rates in supercritical CO2 environment. International Journal of Chemical Kinetics, 51, 42-48.
WANG, H., YOU, X., JOSHI, A. V., DAVIS, S. G., LASKIN, A., EGOLFOPOULOS, F. & LAW, C. 2007. USC Mech Version II. High-Temperature Combustion Reaction Model of H2/CO/C1-C4 Compounds.

