-
What are the combustion and flue gas properties of Blast Furnace Gas (BFG)?
Date posted:
-
-
Post Author
dev@edge.studio
1. Introduction
The typical fuel gases used in integrated iron and steelworks are listed in CF62 and introduced in more detail in CF100. This group of industrial fuel gases includes [GLOSS]Blast Furnace Gas[/GLOSS] or [GLOSS]BFG[/GLOSS].
Blast Furnace Gas BFG is produced as a by-product from pig iron production in [GLOSS]blast furnaces[/GLOSS]. Pig iron, or “[GLOSS]hot metal[/GLOSS]” is a base material for steel production in a basic oxygen furnace or electric arc furnace.
Blast Furnace Gas is a low [GLOSS]calorific value[/GLOSS]fuel gas, which can be used as gaseous fuel in various applications inside or outside the iron making plant. A significant amount of Blast Furnace Gas is used for the production of hot blast in blast furnace stoves.
The production, fuel properties, and applications of BFG are described in associated Combustion File numbers 218, 242 and 244 respectively. The present CF concentrates on the combustion and flue gas properties of BFG.
2. Combustion and flue gas properties of Blast Furnace Gas
Combustion properties that are important when Blast Furnace Gas is fired in industrial furnaces and boilers are:
· The [GLOSS]stoichiometric air requirement[/GLOSS],
· The [GLOSS]adiabatic flame temperature[/GLOSS],
· The [GLOSS]flammability limits[/GLOSS], [GLOSS]auto-ignition temperature[/GLOSS] and;
· The [GLOSS]flue gas[/GLOSS] composition.
Stoichiometric Air Requirement
To burn Blast Furnace Gas under stoichiometric conditions, the air requirement varies between 0.5 mo3/mo3 and 0.8 mo3/mo3. This is a very small air requirement (compared with natural gas: 8.5 mo3/mo3 to 10.0 mo3/mo3) since Blast Furnace gas consists for a considerable part of inert gases (nitrogen, carbon dioxide and water vapour).
The main combustible species in Blast Furnace Gas are carbon monoxide and hydrogen. The specific air requirement to combust these bi-atom molecules is also small compared to the specific air requirement to combust CH4.
Adiabatic Flame Temperature
Adiabatic flame temperature is a good indicator of the heating effectiveness of a fuel, particularly in high temperature processes. Due to the large amounts of inert species in Blast Furnace Gas, the adiabatic flame temperature at stoichiometric conditions is relatively low: 1200 – 1400 °C. Therefore pure blast furnace gas is not suitable as fuel gas for high temperature processes without some form of benefication (CF 249).
Flammability Limits
A Blast Furnace Gas/air mixture at ambient temperature and pressure is explosive if the concentration of Blast Furnace Gas in the mixture is 30 – 70 vol.% [4]. The flammability limits of Blast Furnace Gas are shifted to the rich side compared to the flammability limits of natural gas (6.5 – 16.8 vol.%). This is due to the high amount of inert gases in Blast Furnace Gas. Since the amount of hydrogen in Blast Furnace Gas is strongly dependent on the amount of powder coal injection in the bottom of the blast furnace (see CF 242) the lower flammability limit may vary between 30 and 55 vol.%. The upper flammability limit is less dependent on the amount of hydrogen in the Blast Furnace Gas.
The Auto-ignition Temperature
The auto-ignition temperature of a gas mixture is determined by the lowest auto-ignition temperature of one of the species in the gas mixture. In general, this is the hydrocarbon component with the largest molecule or hydrogen. In a Blast Furnace Gas – air mixture, the auto-ignition temperature of hydrogen determines the auto-ignition temperature. This is 400°C, which is relatively low compared to the auto-ignition temperature of natural gas: 617°C [3].
Flue Gas Composition
In Table 1 the flue gas composition of a stoichiometric Blast Furnace Gas flame, assuming complete combustion, is presented. The plants in the table refer to the same plants as indicated in CF 242.
|
Component |
|
Plant 1 |
Plant 2 |
Plant 3 |
Plant 4 |
Plant 5 |
Plant 6 |
Average |
SD |
|
Carbon Dioxide |
% v/v |
30.6 |
25.4 |
23.7 |
27.2 |
26.2 |
26.1 |
26.5 |
2.3 |
|
Nitrogen |
% v/v |
63.1 |
66.8 |
65.9 |
66.2 |
66.2 |
67.1 |
65.9 |
1.5 |
|
Water Vapour |
% v/v |
5.4 |
7.4 |
10 |
6.2 |
7.2 |
6.4 |
7.1 |
1.6 |
|
Argon |
% v/v |
0.9 |
0.4 |
0.5 |
0.4 |
0.4 |
0.4 |
0.5 |
0.2 |
Table 1 Flue gas properties of stoichiometric Blast Furnace Gas flames, assuming complete combustion.
An overview of the gas properties, combustion and flue gas properties of a typical Blast Furnace Gas is shown in Table 2.
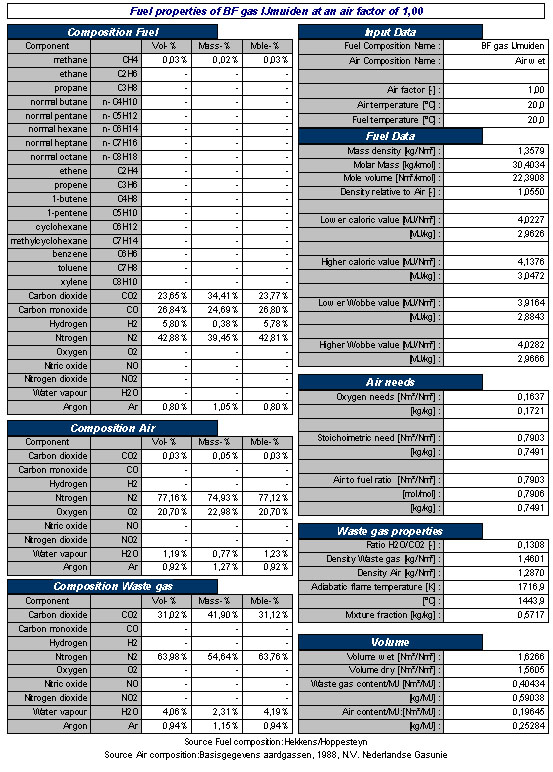
Table 2. Typical example of properties of Blast Furnace Gas and combustion related properties at stoichiometric combustion
Sources
[1] American Iron and Steel Institute, www.steel.org
[2] Ricketts, J.A., How a blast furnace works, Ispat Inland Inc.
[3] N.V. Nederlandse Gasunie, Physical properties of natural gases, Groningen, Netherlands, 1980.
[4] Zabetakis, M.G., Flammability characteristics of combustible gases and vapors, Bulletin 627, Bureau of Mines, 1965.
[5] Informatie Chemische Stoffen, werkinstructiekaart oxygas, Corus, 2002.
