-
CO2 adsorption in shales under CO2 dissolution-induced acidic conditions
Date posted:
-
-
Post Author
Tracey Biller
-
A new study contributes to a better understanding of the mechanisms of CO2 trapping in deep saline aquifers.
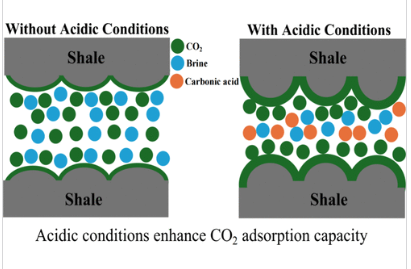
Despite extensive research on CO2 adsorption onto shale, the impact of acidic conditions within shale–CO2–brine systems has received limited attention. A new study experimentally investigates the adsorption of CO2 on three different shales under acidic conditions using a volumetric method and employing molecular dynamics simulation to further validate the experimental findings.
Illite was used as the representative clay mineral due to its substantial presence in shale formations.
The results showed that CO2 adsorption on shale increases under acidic conditions, irrespective of the shale composition. Specifically, the level of CO2 adsorption increased from 2.968 to 3.475 mmol/g in Shale-1, from 3.325 to 4.650 mmol/g in Shale-2, and from 3.573 to 4.852 mmol/g in Shale-3 at 1100 psia and 313 K.
The observed increase in the level of CO2 adsorption suggests that the shale caprock’s ability to retain CO2 decreases under acidic conditions. This reduction in the trapping capacity heightens the risk of CO2 leakage. Additionally, excessive CO2 adsorption can induce shale swelling, elevating pore pressure and increasing the likelihood of fracture, which could further compromise caprock integrity.
